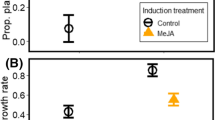
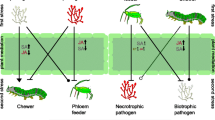
Summary
Two species of lepidopteran herbivores, Manduca sexta (Sphingidae) and Trichoplusia ni (Noctuidae), were reared on synthetic diet containing either the alkaloid nicotine or the flavonoid rutin. Survival and pupal weight of the specialist M. sexta did not differ when larvae were reared on diet containing nicotine or rutin. In contrast, the generalist T. ni did not survive on diet containing 0.125% nicotine or greater, whereas larvae survived on all concentrations of rutin. These data demonstrate that the alkaloid nicotine is inhibitory toward generalist, but not specialist herbivores, whereas the flavonoid rutin has no effect on specialist herbivores and limited effects on generalist herbivores. Five species of Pseudomonas bacterial pathogens: P. syringae, P. syringae pv. angulata, P. syringae pv. tabaci, P. fluorescens, and P. solanacearum were grown on nutrient agar containing nicotine or rutin at concentrations ranging from 0.0 to 1.0% wet weight in 0.1% intervals. No species of Pseudomonas grew at concentrations greater than 0.5% nicotine when 106 colony forming units (cfu) were used, but growth occurred at all concentrations of rutin when 102 cfu were used. These data indicate that nicotine was inhibitory to growth of both herbivores and pathogens, suggesting that certain plant secondary chemicals with high toxicity are of a generalized nature and affect multiple species. Differences in the sensitivity of organisms to allelochemicals such as generalist or specialist can make it appear that specific allelochemicals affect specific organisms, when in fact it is the tolerance of the organism to the plant chemical that is responsible. In four separate studies, the growth of M. sexta, T. ni and Helicoverpa zea was significantly lower on plants inoculated with P. solanacearum. Alteration in leaf quality by P. solanacearum was due to either reductions in leaf nutrients or increases in allelochemicals. We speculate that localized or systemic induction by both herbivores and pathogens can cause changes in leaf quality, effecting each other's subsequent colonization. The generalized nature of plant secondary compounds and potential reciprocal effects on induction by both species suggests that herbivores and pathogens may affect plant quality through induction and diffuse interactions of disparate species can alter the community of organisms colonizing a plant.
Similar content being viewed by others
References
Afek U, Sztejnberg A (1988) Accumulation of scoparone, a phytoalexin associated with resistance of citrus to Phytophthora citrophthora. Phytopathology 78:1678–1682
Baldwin I (1988a) Short-term damage-induced increases in tobacco alkaloids protect plants. Oecologia 75:367–370
Baldwin I (1988b) Damage induced alkaloids in tobacco: pot bound plants are not inducible. J Chem Ecol 47:111–123
Baltensweiler W, Benz G, Delucchio V (1977) Dynamics of larch bud moth populations. Annu Rev Entomol 22:79–100
Barbosa P, Krischik VA (1987) Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth, Lymantria dispar. Am Nat 130:53–69
Benz G (1977) Insect-induced resistance as a means of self defense in plants. Eucarpia/IOBC Work. Group B. Resistance, insects, mites. Bull CROP 1977/1978, pp 155–159
Bernays EA, Chapman RF (1978) Deterrent chemicals as a basis of oligophagy in Locusta migratoria. Ecol Entomol 2:1–18
Cantot P, Papineau J (1983) Discrimination des lupins a basse teneur en alkaloides par les adultes de Sitona lineatus (L.) (Col., Curculinodae). Agronomie 3:937–940
Carroll CR, Hoffman CA (1980) Chemical feeding deterrent mobilized in response to insect herbivory and counteradaptation by Epilachna tredecimnota. Science 209:414–416
Caruso F, Kuc J (1977) Protection of watermelon and muskmelon against Colletotrichum lagenurium by Colletotrichum larenarium. Phytopathology 67:1285–1289
Caruso F, Kuc J (1979) Induced resistance of cucumber to anthracnose and angular leaf spot by Pseudomonas lachrymans and Colletotrichum lagenarium. Physiol Plant Pathol 14:191–201
Chan BC, Waiss AC, Lukefar M (1978) Condensed tannin, and antibiotic chemical from Gossypium hirsutum. J Insect Physiol 24:113
Chapman RF (1974) The chemical inhibition of feeding by phytophagous insects. Bull Entomol Res 64:339–363
Clayton EE (1942) Resistance of tobacco to bacterial wilt. J Agric Res 65:547–554
Cohen Y, Kuc J (1981) Evaluation of systemic resistance to blue mold induced in tobacco leaves by prior stem inoculation with Peronospora hyoscyami tabacina. Phytopathology 71:783–787
Coleman J, Jones CG (1991) A phytocentric view of phytochemical induction by herbivores. In: Tallamy D, Raupp M (eds) Phytochemical induction by herbivores. Wiley, New York (in press)
Cruickshank I, Mandryk M (1960) The effect of stem infestation of tobacco with Peronospora tabacina on foliage infection to blue mold. J Aust Inst Agric Sci 26:369–372
Davis KR, Darvill AG, Albrsheim P (1986) Host-pathogen interactions. XXXI. Several biotic and abiotic elicitors act synergistically in the induction of phytoalexin accumulation in soybean. J Plant Mol Biol 6:23–32.
Deall MW, Cole JS (1986) A comparative study of the pathogenicity and epidemiology of strains of Pseudomonas syringae var. that cause wildfire and angular leaf spot diseases of tobacco in Zimbabwe. Plant Pathol 35:74–81
Denno RF, McClure MS (1983) Variable plants and herbivores in natural and managed systems. Academic Press, New York
Devitt BD, Philogene BJR, Hinks CF (1980) Effects of veratrine, berberine, nicotine, and atropine on developmental characteristics and survival of the dark-sided cutworm Euxoa messeria (Lepidoptera: Noctuidae). Phytoprotection 61:88–102
Dowler WM, Peterson DH (1980) Bacteriological techniques for Pseudomonas. USDA Publication of Clemson University, Clemson, S.C.
Edwards PJ, Wratten SD (1980) Ecology of insect-plant interactions. Arnold, London
Edwards PJ, Wratten SD (1983) Wound induced defences in plants and their consequences for patterns of insect grazing. Oecologia 59:88–93
Elliger CA, Chan BC, Waiss AC Jr (1980) Flavonoids as larval growth inhibitors: structural faverning toxicity. Naturwissenschaften 67:358–360
Elliger CA, Wong Y, Chan BG, Waiss AC Jr (1981) Growth inhibitors in tomato (Lycopersicon) to tomato fruitworm (Heliothis zea). J Chem Ecol 7:753–758
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 57:565–581
Feeny P (1975) Biochemical coevolution between plants and their insect herbivores. In: Gilbert LE, Raven PH (eds) Coevolution of animals and plants. University of Texas Press, Austin, Tex, pp 3–19
Feeny P (1976) Plant apparency and chemical defense. In: Wallace J, Mansell R (eds) Biochemical interactions between plants and insects. (Recent advances in phytochemistry, vol. 10) Plenum, New York, pp 1–14
Fowler SV, Lawton JH (1985) Rapidly induced defenses and talking trees: the devil's advocate position. Am Nat 126:181–195
Fraenkel GS (1959) The raison d'etre of secondary plant substances. Science 125:1466–1470
Goth R, Krischik VA, Barbosa P (1987) Interactions among plant allelochemicals, plant pathogens, and insect herbivores. Phytopathology 77:896
Green TR, Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175:776–777
Green TR, Ryan CA (1973) Wound-induced proteinase inhibitor in tomato leaves. Plant Physiol 51:19–21
Guedes MEM, Richmond S, Kuc J (1980) Induced systemic resistance to anthracnose in cucumber as influenced by the location of the inducer inoculation with Colletotrichum lagenarium and onset of flowering and fruiting. Physiol Plant Pathol 17:229–233
Harborne JB (1979) Flavonoid pigments. In: Rosenthal GA, Janzen D (eds) Herbivores: their interaction with secondary plant metabolites. Academic, New York, pp 619–655
Hardwiger LA, Webster DM (1984) Phytoalexin production in five culivars of peas differentially resistant to three races of Pseudomonas syringae pv. pisi. Phytopathology 74:1312–1314
Hare RC (1966) Physiology of resistance to fungal diseases in plants. Bot Rev 32:95–137
Hare JD (1983a) Seasonal variation in plant-insect associations: utilization of Solanum dulcamara by Leptinotarsa decemlineata. Ecology 64:345–361
Hare JD (1983b) Manipulation of host suitability for herbivore pest management. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 655–680
Hare JD, Andreadis TG (1983) Variation in the susceptibility of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) when reared on different host plants to the fungal pathogen, Beauveria bassiana in the field and laboratory. Environ Entomol 12:1891–1896
Hart SV, Kogan M, Paxton JD (1983) Effect of soybean phytoalexins on the herbivorous insects Mexican bean beetle and soybean looper. J Chem Ecol 9:657–673
Hartley SE, Lawton JH (1987) Effects of different types of damage on the chemistry of birch foliage, and the response of birchfeeding insects. Oecologia 74:432–437
Haukioja E, Niemela P (1977) Retarded growth of a geometrid larva after mechanical damage to leaves of its host tree. Ann Zool Fenn 14:48–52
Jenns AE, Kuc J (1979) Graft transmission of systemic resistance of cucumber to anthracnose induced by Colletotrichum lagenarium and tobacco necrosis virus. Phytopathology 69:753–756
Jermy T (1976) Insect-host plant relationship-coevolution or sequential evolution? Symp Biol Hung 16:109–113
Jermy T (1984) Evolution of insect-host plant relationships. Am Nat 124:609–630
Jones CG (1984) Microorganisms as mediators of plant resource exploitation by insect herbivores. In: Price PW, Slobodchikoff CN, Gaud WS (eds) A new ecology: novel approaches to interactive systems. Wiley, New York, pp 54–99
Jones CG, Coleman JS (1990) Plant “stress” and insect herbivory: toward an integrated perspective. In: Mooney HA, Winner W, Pell E (eds) An integrated approach to the study of environmental stress on plant growth. Academic Press, New York (in press)
Karban R (1987) Environmental conditions affecting the strength of induced resistance against mites in cotton. Oecologia 73:414–419
Karban R, Carey JR (1984) Induced resistance of cotton seedlings to mites. Science 225:53–54
Karban R, Adamchak R, Schnathorst WC (1987) Induced resistance and interspecific competition between spider mites and a vascular wilt fungus in cotton plants. Science 235:678–680
Keen NT, Brueger B (1977) Phytoalexins and chemicals that elicit their production in plants. In: Hedin PA (ed) Host plant resistance to pests. Am Chem Soc Symp Ser 62, Washington, DC, pp 1–26
Kelman A (1953) The bacterial wilt caused by Pseudomonas solanacearum, N C Agric Exp St Tech Bull 99
Kelman A (1954) The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in tetrazolium medium. Phytopathology 44:693–695
Klement Z (1977) Methods for the rapid detection of pathogenicity of phytopathogen Pseudomonads. Nature 199:299–300
Kogan M, Paxton J (1983) Natural inducers of plant resistance to insects. In: Hedin PA (ed) Plant resistance to insects. ACS Symp Serv 208, Washington, D.C., pp 153–171
Kopp M, Rouster J, Fritig B, Darvill A, Albersheim P (1989) Hostpathogen interactions. XXXII. A fungal glucan preparation protects nicotianae against infection by viruses. Plant Physiol 90:208–216
Krischik VA (1991) Specific or generalized, plant defense: Reciprocal interactions between herbivores and pathogens. In: Barbosa P, Krischik VA, Jones CJ (eds) Microbial mediation of plantherbivore interactions. J. Wiley and Sons, New York, pp 211–229
Krischik VA, Denno RF (1983) Individual, populational, and geographic patterns in plant defense. In: Denno RF, McClure M (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 463–512
Krischik VA, Barbosa P, Reichelderfer CF (1988) Three trophic interactions: allelochemicals, Manduca sexta, and Bacillus thuringiensis. Environ Entomol 17:476–482
Kuc J (1966) Resistance of plants to infectious agents. Annu Rev Microbiol 20:337–364
Kuc J (1972) Phytoalexins. Annu Rev Phytoptahol 10:207–232
Kuc J (1982) Plant immunization-mechanisms and practical implications. In: Wood RKS, Tiamos E (eds) Active defensive mechanisms in plants. Plenum. New York, pp 157–178
Kuc J, Richmond S (1977) Aspects of the protection of cucumber against Colletotrichum lagenarium by Colletotrichum lagenarium. Phytopathology 67:533–536
Levin DA (1976) The chemical defenses of plants to pathogens and herbivores. Ann Rev Ecol Syst 7:121–159
Levinson HZ (1976) The defensive role of alkaloids in insects and plants. Experientia 32:408–411
Lyon GD (1984) Comparisons between phytoalexin concentrations and the extent of rotting of potato tubers inoculated with Erwinia carotovora subsp. atroseptica, Erwinia carotovora subsp. Carotovora or Erwinia chrysanthemi. Z Phytopathol 111:236–243
McKey D (1974) Adaptive patterns in alkaloid physiology. Am Nat 108:305–320
McKey D (1979) The distribution of secondary compounds within plants. In: Rosenthal GA, Janzen DH (eds) Herbivores: their interaction with secondary plant metabolites. Academic Press, New York, pp 56–133
Metcalf CL, Flint WP (1962) Destructive and useful insects: their habits and control. McGraw-Hill, New York
Miller JS, Feeny P (1983) Effects of benzylisoquinoline alkaloids on the larvae of polyphagous Lepidoptera. Oecologia 58:332–339
Mitchell BK, Harrison GD (1985) Effects of Solanum glycoalkaloids on chemosensilla in the Colorado potato beetle. A mechanism of feeding deterrence? J Chem Ecol 11:73–83
Munyemana PC (1986) Essai de preservation du haricot (Phaseolus vulgaris L) contre Acanthoscelides obtectus Say en utilisant les produits d'origine vegetale. Memoir. Faculty of Agronomy, National University of Ruwanda
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores: their interaction with secondary, plant metabolites. Academic Press, New York, pp 3–54
Rhoades DF (1983a) Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows. In: Hedin P (ed) Mechanisms of plant resistance to insects. ACS Symp Ser 208, pp 55–68
Rhoades DF (1983b) Herbivore population dynamics and plant chemistry. In: Denno RF, McClure MS. Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 155–220
Rhoades DF, Cates RG (1976) Toward a general theory of plant antiherbivore chemistry. In: Wallace J, Mansell R (eds) Biochemical interactions between plants and insects. Recent advances in phytochemistry, vol 10. Plenum, New York, pp 168–213
Roddick JG (1974) The steroidal glycoalkaloid-tomatine. Phytochemistry 13:9–25
Rosenthal GA, Janzen DH (eds) (1979) Herbivores: their interaction with secondary plant metabolites. Academic Press, New York
Ryan CA (1979) Proteinase as inhibitors. In: Rosenthal GA, Janzen DH. Herbivores: their interaction with secondary plant metabolites. Academic Press, New York, pp 599–618
Ryan CA (1983) Insect-induced chemical signals regulating natural plant protection responses. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 43–60
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–150
Sequeira L (1983) Mechanisms of induced resistance in plants. Annu Rev Microbiol 37:51–79
Sinden SL, Goth RW, O'Brien MJ (1973) Effect of potato alkaloids on the growth of Alternaria solani and their possible role as resistance factors in potatoes. Phytopathology 63:303–307
Sinden SL, Schalk JM, Stoner AK (1978) Effects of daylength and maturity of tomato plants on tomatine content and resistance to the Colorado potato beetle. J Am Soc Hort Sci 103:596–600
Sisson VA, Saunders JA (1982) Alkaloid composition of the USDA tobacco (Nicotiana tabacum L.) introduction collection. Tob Sci 26:117–120
Sisson VA, Saunders JA (1983) Catalog of the tobacco introductions in the U.S. Department of Agriculture's tobacco germplasm collection (Nicotiana tubacum). Agricultural Research Service, USDA, ARM-5-27, pp 1–27
Stolle K, Zook M, Shain L, Hebard F, Kuc J (1988) Restricted colonization by Peronospora tabacina and phytoalexin accumulatin in immunized tobacco leaves. Phytopathology 78:1193–1197
Swain T (1974) Biochemical evolution in plants. In: Florkin M, Stotz EH (eds) Comprehensive biochemistry, vol 29A. Elsevier, Amsterdam, pp 105–302
Swain T (1977) The effect of plant secondary products on insect plant co-evolution. Annu Rev Plant Physiol 28:479–501
Tallamy DW (1985) Squash beetle feeding behavior: an adaptation against induced cucurbit defenses. Ecology 66:1574–1579
Tallamy DW, Krischik VA (1989) Variation and function of cucurbitacins in Cucurbita: an examination of current hypotheses. Am Nat 133:766–786
Vandenberg P, Matzinger DF (1970) Genetic diversity and heterosis in Nicotiana III. Crosses among tobacco introductions and flue cured varieties. Crop Sci 10:437–440
Van Puyvelde L (1986) Etude chimique et pharmacalogique d'umuravumba; Iboza riparia (Hochst) N.E. Br. (Lamiacees). Rev Ruwandaise Sci 1:1–10
Waller GR, Nowacki EK (1978) Alkaloid biology and metabolism in plants. Plenum, New York.
Wallner WE, Walton GS (1979) Host defoliation: a possible determinant of gypsy moth population quality. Ann Entomol Soc Am 72:62–67
Whittaker RH, Feeny PP (1971) Allelochemicals: chemical interactions between species. Science 171:757–770
Wink M (1984) Chemical defense of Leguminosae: are quinolizidine alkaloids part of the antimicrobial system of lupines? Naturforsch 39:548–552
Yamamoto RT, Graenkel G (1960) Assay of the principal gustatory stimulant for the tobacco hornworm, Protoparce sexta, from solanaceous plants. Ann Entomol Soc Am 53:499–503
Yang RSH, Guthrie FE (1969) Physiological responses of insects to nicotine. Ann Entomol Soc Am 62:141–146
Zucker WV (1983) Tannins: does structure determine function? An ecological perspective. Am Nat 123:335–365
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krischik, V.A., Goth, R.W. & Barbosa, P. Generalized plant defense: effects on multiple species. Oecologia 85, 562–571 (1991). https://doi.org/10.1007/BF00323769
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00323769