Abstract
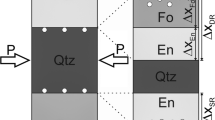
Two experiments using cylindrical samples of a dolomite-quartz rock were carried out in a conventional hydrothermal apparatus for the forward reaction: 1 dolomite + 2 quartz = 1 diopside + 2 CO2, in order to compare the mechanism and the kinetics with results from experiments using mineral powders of dolomite and quartz at the same P-T-X conditions. Experimental conditions were as follows: total pressure 500 MPa; temperature 680° C (overstepping 65° C); CO2 content of the fluid phase, consisting of carbon dioxide and water, was nearly 90 mol%; the fluid/rock ratio was 1:37, and the H2O/rock ratio was about 1:740; run duration was 92 days. Scanning electron microscope (SEM) examination of a polished axial section of the rock cylinders after the run, using back-scattered electrons (BSE), shows that the reaction produced corona textures. The diopside crystals nucleate and grow exclusively on dolomite surfaces adjacent to quartz grains, i.e. in regions where there is intimate contact between the reactants. The dolomite matrix, in contrast, is diopside free. A concept of microsystems is used to compare directly the rock cylinder results with those from runs done with mineral powders. The microsystems, which consist of quartz, dolomite and diopside, are connected by the intergranular space which is filled by the fluid phase. The SEM analysis of the rock cylinders indicates a dissolution-crystallization mechanism operating in the microsystems; this is consistent with the results of experiments using dolomite quartz powders (Lüttge et al. 1989). It can be demonstrated that reaction kinetics in mineral powder runs are interface controlled as long as the newly formed diopside crystals do not cover the dolomite surfaces completely (Lüttge and Metz 1991 c). This result is applicable to each microsystem of the rock cylinder, since the reaction mechanism and the resulting textures are the same in both kinds of experiments. The reaction is much slower outside the microsystems, i.e. in the dolomite matrix but in the close vicinity of the quartz grains. At these places, the reaction is controlled by the transport of Si-species in the CO2-rich fluid phase filling the intergranular space. The reaction is absent in quartz-free regions of the dolomite matrix. Calculations and measurements of the extent of reaction progress in both kinds of experiments give results of the same order of magnitude: the conversion, and therefore the reaction rate, differs by less than a factor of two. The conclusion is that there are no differences, in principle, concerning mechanisms, rate controls, rates, and resulting textures between rock cylinder experiments, and mineral powder experiments.
Similar content being viewed by others
References
Dachs E, Metz P (1988) The mechanism of the reaction 1 tremolite+3 calcite+2 quartz=5 diopside+3 CO2+1 H2O: results of powder experiments. Contrib Mineral Petrol 100:542–551
Gottschalk M (1990) Intern konsistente thermodynamische Daten im System SiO2−Al2O3−CaO−MgO−K2O−Na2O−H2O−CO2. PhD dissert, Univ. Tübingen, Germany
Heinrich W, Metz P, Bayh W (1986) Experimental investigation of the mechanism of the reaction: 1 tremolite+11 dolomite =8 forsterite+13 calcite+9 CO2+1 H2O. Contrib Mineral Petrol 93:215–221
Heinrich W, Metz P, Gottschalk M (1989) Experimental investigation of the kinetics of the reaction 1 tremolite+11 dolomite =8 forsterite+13 calcite+9 CO2+1 H2O. Contrib Mineral Petrol 102:163–173
Heuss-Assbichler S, Masch L (1991) Microtextures and reaction mechanisms of carbonate rocks: a comparison between the thermoaureoles of Ballachulish and Monzoni (N Italy). In: Voll G, Töpel J, Pattison DRM, Seifert F (eds) Equilibrium and kinetics in contact metamorphism. Springer, Berlin Heidelberg New York, pp 229–249
Käse HR, Metz P (1980) Experimental investigation of the metamorphism of siliceous dolomites. Contrib Mineral Petrol 73:151–159
Kerrick DM (ed) (1991) Contact metamorphism. (Reviews in Mineralogy, vol 26) Mineral Soc Am, Washington, DC
Kretz R (1983) Symbols for rock-forming minerals. Am Mineral 68:277–279
Loomis TP (1976) Irreversible reactions in high-grade metapelitic rocks. J Petrol 17:559–588
Lüttge A, Metz P (1991a) Rate-limiting processes in the course of the reaction: 1 dolomite+2 quartz=1 diopside+2 CO2 (abstract). Terra Abstr 3:434
Lüttge A, Metz P (1991 b) Gesteinszylinderexperimente zum Mechanismus und zur Kinetik der Reaktion: 1 Dolomit +2 Quarz=1 Diopsid+2 CO2 (abstract). Beih Eur J Mineral 3/1:173
Lüttge A, Metz P (1991 c) Mechanism and kinetics of the reaction: 1 dolomite+2 quartz=1 diopside+2 CO2 investigated by powder experiments. Can Mineral 4:803–821
Lüttge A, Metz P, Rehländer R (1987) Concepts of the mechanism of decarbonation reactions in siliceous carbonates (abstract). Terra cognita 7:253
Lüttge A, Dachs E, Metz P (1989) Mechanism of diopside-formation in siliceous dolomites. 2. Results of experiments with hot pressed and natural starting materials (abstract). Terra Abstr 1:148
Matthews A (1980) Influence of kinetics and mechanism in metamorphism: a study of albite crystallization. Geochim Cosmochim Acta 44:387–402
Matthews A (1985) Kinetics and mechanisms of the reaction of zoisite to anorthite under hydrothermal conditions: reaction phenomenology away from the equilibrium region. Contrib Mineral Petrol 89:110–121
Roedder E (1984) Fluid Inclusions. (Reviews in mineralogy, vol 12) Mineral Soc Am, Washington DC
Rubie DC (1986) The catalysis of mineral reactions by water and restrictions on the presence of aqueous fluid during metamorphism. Mineral Mag 50:399–415
Schramke JA, Kerrick DM, Lasaga AC (1987) The reaction muscovite +quartz=andalusite+K-feldspar+water. 1. Growth kineties and mechanism. Am J Sci 287:517–559
Tanner SB, Kerrick DM, Lasaga AC (1985) Experimental kinetic study of the reaction: calcite+quartz=wollastonite+carbon dioxide, from 1 to 3 kilobars and 500 to 850° C. Am J Sci 285:577–620
Thompson AB, Rubie DC (eds) (1985) Metamorphic reactions. (Advances in physical Geochemistry 4) Springer, Berlin Heidelberg New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lüttge, A., Metz, P. Mechanism and kinetics of the reaction: 1 dolomite + 2 quartz = 1 diopside + 2 CO2: a comparison of rock-sample and of powder experiments. Contr. Mineral. and Petrol. 115, 155–164 (1993). https://doi.org/10.1007/BF00321217
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00321217