Summary
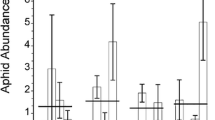
We investigated the effects of genotype, habitat, and seasonal variation on production of the iridoid glycosides, aucubin and catalpol, in leaves of the common weed Plantago lanceolata. Two genotypes, one each from a lawn and an adjacent abandoned hayfield population, were clonally replicated in the greenhouse, and then planted back into the two habitats. One quarter of the plants from each treatment were harvested on each of four dates, at approximately two-week intervals. Over the course of the growing season, and in both habitats, we found a significant increase in the concentration of both aucubin and catalpol in P. lanceolata leaves. The genotypes differed in their response to environmental variation, both in time and between sites, as indicated by significant genotype x date and genotype x site interactions. Early in the season, habitat (lawn or field) had a greater effect on iridoid glycoside concentration than did plant genotype, but later in the season, plant genotype was more influential in determining the iridoid glycoside concentration. Thus, the relative palatability of Plantago genotypes to specialist and generalist herbivores may vary in time and space.
Similar content being viewed by others
References
Belofsky G, Bowers MD, Janzen S, Stermitz FR (1989) Iridoid glycosides of Aureolaria flava and their sequestration by Euphydryas phaeton butterflies. Phytochemistry 28:1601–1604
Berenbaum M (1981) Patterns of furanocoumarin production and insect herbivory in a population of wild parsnip (Pastinaca sativa L). Oecologia 49:236–244
Berenbaum MR, Zangerl AR, Nitao JK (1986) Constraints on chemical coevolution: wild parsnips and the parsnip webworm. Evolution 40:1215–1228
Bowers MD (1980) Unpalatability as a defense strategy of Euphydryas phaeton (Lepidoptera: Nymphalidae). Evolution 34:586–600
Bowers MD (1983) Iridoid glycosides and larval hostplant specificity in checkerspot butterflies (Euphydryas, Nymphalidae). J Chem Ecol 9:475–493
Bowers MD (1984) Iridoid glycosides and hostplant specificity in larvae of the buckeye butterfly, Junonia coenia (Nymphalidae). J Chem Ecol 10:1567–1577
Bowers MD (1992) The evolution of unpalatability and the cost of chemical defense in insects. In: Evolutionary perspectives on the chemical ecology of insects. Isman MB, Roitberg B (eds) Chapman and Hall, New York (in press)
Bowers MD, Collinge SK (1992) The fate of ingested iridoid glycosides in different life stages of the buckeye, Junonia coenia (Nymphalidae). J Chem Ecol 18:817–831
Bowers MD, Puttick GM (1986) The fate of ingested iridoid glycosides in lepidopteran herbivores. J Chem Ecol 12:169–178
BowersMD, Puttick GM (1988) The response of generalist and specialist insects to qualitative allelochemical variation. J Chem Ecol 14:319–334
Bowers MD, Puttick GM (1989) Iridoid glycosides and insect feeding preferences: gypsy moths (Lymantria dispar, Lymantriidae) and buckeyes (Junonia coenia Nymphalidae). Ecol Entomol 14:247–256
Bowers MD, Stamp NE (1992) Chemical variation within and between individuals of Plantago lanceolata (Plantaginaceae). J Chem Ecol 18:985–995
Briske DD, Camp BJ (1982) Water stress increases alkaloid concentration in threadleaf groundsel (Senecio longilobus), Weed Sci 30:106–114
Fajer ED, Bowers MD, Bazzaz FA (1992) The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: a test of the carbon/nutrient balance hypothesis. Am Nat (in press)
Gardner D, Stermitz FR (1988) Host-plant utilization and iridoid glycoside sequestration by Euphydryas anicia (Lepidoptera: Nymphalidae) J Chem Ecol 14:2147–2168
Gershenzon, J (1984) Changes in the levels of plant secondary metabolites under water and nutrient stress. Rec Adv Phytochem 18:273–320
Hatcher PE (1990) Seasonal and age-related variation in the needle quality of five conifer species. Oecologia 85:200–212
Janzen D, Waterman P (1984) A seasonal census of phenolics, fibre and alkaloids in foliage of forest trees in Costa Rica: some factors influencing their distribution and relation to host selection by Sphingidae and Saturniidae. Biol J Linn Soc 21:439–454
Johnson ND, Chu CC, Ehrlich PR, Mooney HA (1984) The seasonal dynamics of leaf resin, nitrogen, and herbivore damage in Eriodictyon californicum and their parallels in Diplacus aurantiacus. Oecologia 61:398–402
Kearsley MJC, Whitham TG (1989) Developmental changes in resistance to herbivory: implications for individuals and populations. Ecology 70:422–434
Kooi RE, Van de Water TPM, Herrebout WM (1991) Food acceptance by a monophagous and an oligophagous insect in relation to seasonal changes in host plant suitability. Entomol Exp Appl 59:111–122
Krischik VA, Denno RF (1983) Individual, population, and geographic patterns in plant defense. In: Variable plants and herbivores in natural and managed systems. Denno RF, McClure MS (eds) Academic Press, Orlando. pp 463–512
Lechowicz MJ (1984) Why do temperate deciduous trees leaf out at different times? adaptation and ecology of forest communities. Am Natur 124:821–842
Lincoln DE, Langenheim JH (1979) Variation of Satureja douglasii monoterpenoids in relation to light intensity and herbivory. Biochem Syst Ecol 7:289–298
Lincoln DE, Langenheim JH (1981) A genetic approach to monoterpenoid variation in Satureja douglasii. Biochem Syst Ecol 9:153–161
Lindroth RL, Batzli GO, Seigler DS (1986) Patterns in the phytochemistry of three prairie plants. Biochem Syst Ecol 14:597–602
Lindroth RL, Hsia MT, Scriber JM (1987) Seasonal Patterns in the phytochemistry of three Populus species. Biochem Syst Ecol 15:681–686
Mabry TJ (1970) Infraspecific variation of sesquiterpene lactones in Ambrosia (Compositae): applications to evolutionary problems at the population level. In: Harborne JB (ed) Phytochemical phylogeny. Academic Press, London, pp 269–300
Maddox GD, Cappuccino N (1986) Genetic determination of plant susceptibility to an herbivorous insect depends on environmental context. Evolution 40:863–866
Marquis RJ (1983) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539
Marquis RJ (1990) Genetic variation in Piper arieianum (Piperaceae) by a multispecies assemblage of herbivores. Evolution 44:104–120
Mattson WJ, Haack RA (1987) The role of drought in outbreaks of plant-eating insects. BioScience 37:110–118
Mauffette Y, Oechel WC (1989) Seasonal variation in leaf chemistry of the coast live oak Quercus agrifolia and implications for the California oak moth Phryganidia california. Oecologia 79:439–445
McKey D (1979) The distribution of secondary compounds within plants. In: Rosenthal GA, Janzen DH (eds) Herbivores: Their interaction with Secondary Plant Metabolites. Academic Press, New York, pp 55–133
Mihaliak CA, Lincoln DE (1989) Changes in leaf mono- and sesquiterpene metabolism with nitrate availability and leaf age in Heterotheca subaxillaris. J Chem Ecol 15:1579–1588
Mooney HA, Chu CC (1974) Seasonal carbon allocation in Heteromeles arbutifolia, a California evergreen shrub. Oecologia 14:295–306
Nelson CJ, Seiber JN, Brower LP (1981) Seasonal and intraplant variation of cardenolide content in the California milkweed, Asclepias eriocarpa, and the implications for plant defense. J Chem Ecol 7:981–1010
Pereyra PC, Bowers MD (1988) Iridoid glycosides as oviposition stimulants for the buckeye, Junonia coenia (Nymphalidae). J Chem Ecol 14:917–928
Rodman JE, Louda SM (1984) Phenology of glucosinolate concentrations in roots, stems and leaves of Cardamine cordofolia. Biochem Syst Ecol 12:37–46
Schmitt J, Niles J, Wulff RD (1992) Norms of reaction of seed traits to maternal environments in Plantago lanceolata. Am Nat 139:451–466
Simms E, Rausher MD (1989) The evolution of resistance to herbivory in Ipomoea purpurea. II. Natural selection by insects and costs of resistance. Evolution 43:573–585
Strauss SY (1991) The role of plant genotype, environment and gender in resistance to a specialist chrysomelid herbivore. Oecologia 84:111–116
Wagner MR, Clancy KM, Tinus RW (1990) Saasonal patterns in the allelochemicals of Pseudotsuga nenziesii, Picea engelmanii, and Abies concolor. Biochem Syst Ecol 18:215–220
Waterman PG, Mole S (1989) Extrinsic factors influencing production of secondary metabolites in plants. In: Bernays EA (ed) Insect-plant interactions, vol 1, CRC Press, Boca Raton, Florida, pp 107–134
Wu L, Antonovics J (1975) Experimental ecological genetics in Plantago. I. Induction of roots and shoots on leaves for large scale vegetative propagation and metal tolerance testing in P. lanceolata. New Phytol 75:277–282
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deane Bowers, M., Collinge, S.K., Gamble, S.E. et al. Effects of genotype, habitat, and seasonal variation on iridoid glycoside content of Plantago lanceolata (Plantaginaceae) and the implications for insect herbivores. Oecologia 91, 201–207 (1992). https://doi.org/10.1007/BF00317784
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317784