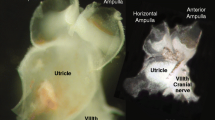
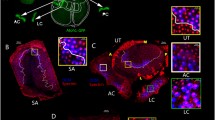
Summary
Early afferent innervation and differentiation of sensory vestibular cells were studied in mouse embryos from gestation day (GD) 13 to 16. Afferent neurites were found as early as GD 13 in the epithelium when there were no clearly differentiated sensory cells. By GD 14 the earliest sensory cells which exhibited short hair bundles at their luminal pole were then contacted by afferent endings at their basal part. On GD 15 nerve endings establishing specialized synaptic contacts, characterized by asymmetrical membrane densities and synaptic bodies, were observed. At this stage, microtubules contacting the presynaptic membranes, as well as coated vesicles were found. On GD 16 the hair cells were multi-afferented and numerous synaptic bodies were found. These results showing a concomitance between the hair cell differentiation and the establishment of nerve contacts are discussed with particular respect to nerv-hair cell interactions during sensory differentiation. This study does not point to a primary induction of vestibular hair cell differentiation by nerve endings, but it is consistent with the possibility that the ingrowth of nerve fibers is one of many factors that influence the differentiation of receptor cells. With respect to synapse formation, it is assumed that the location of synaptic bodies at presynaptic densities is determined by the arrival of afferent nerve endings.
Similar content being viewed by others
References
Altman J (1971) Coated vesicles and synaptogenesis. A developmental study in the cerebellar cortex of the rat. Brain Res 30:311–322
Anniko M, Nordemar H, Van de Water TR (1979) Embryogenesis of the inner ear. I. Development and differentiation of the mammalian crista ampullaris in vitro and in vivo. Arch Oto-Rhino-Laryngol 224:285–299
Dechesne CJ, Sans A, Keller A (1985) Onset and development of neuronspecific enolase immunoreactivity in the peripheral vestibular system of the mouse. Neurosci Lett 61:299–304
Desmadryl G, Raymond J, Sans A, (1985) Histogenesis of the vestibular sensory epithelium in organotypic culture of mouse embryo otocysts: A tritiated thymidine autoradiographic study. Int J Dev Neurosci 3:549–557
Dontenwill M, Roussel G, Zanetta JP (1985) Immunohistochemical localization of a lectin-like molecule, R1, during the postnatal development of the rat cerebellum. Dev Brain Res 17:245–252
Eckenhoff MF, Pysh JJ (1979) Double-walled coated vesicle formations: evidence for massive and transient conjugate internalization of plasma membranes during cerebellar development. J Neurocytol 8:623–638
Eckenhoff MF, Pysh JJ (1983) Conjugate internalization of apposed plasma membranes in mouse olfactory bulb during postnatal development. Dev Brain Res 6:201–207
Favre D, Sans A (1979) Embryonic and postnatal development of afferent innervation in cat vestibular receptors. Acta Otolaryngol (Stockh) 87:97–107
Favre D, Bagger-Sjöback D, Mbiene J-P, Sans A (1986) Freezefracture study of the vestibular hair cell surface during development. Anat Embryol 175:69–76
Friedmann I (1965) The ear. In: Willner EN (ed) Cells and Tissues in Culture, Vol. II. Academic Press, New York London, pp 521–547
Friedmann I, Hodges MG, Riddle PN (1977) Organ culture of the mammalian and avian embryo otocyst. Ann Otol Rhinol Laryngol 86:371–380
Ginzberg R, Gilula N (1979) Modulation of cell junctions during differentiation of the chicken otocyst sensory epithelium. Dev Biol 68:110–129
Ginzberg R, Gilula N (1980) Synaptogenesis in the vestibular sensory epithelium of the chick embryo. J Neurocytol 9:405–424
Gleisner L, Wersäll J (1975) Experimental studies on the nervesensory cell relationship during degeneration and regeneration in ampullar nerves of the frog labyrinth. Acta Otolaryngol (Stockh) Suppl 333:1–28
Hirokawa N (1977) Disappearance of afferent and efferent nerve terminals in the inner ear of the chick embryo after chronic treatment with β-bungarotoxin. J Cell Biol 73:27–46
Hirokawa N (1977) Synaptogenesis in the basilar papilla of the chick. J Neurocytol 7:283–300
Jorgensen JM, Flock A (1976) Non-innervated sense organs of the lateral line: Development in regenerating tail of the salamander (Ambystoma mexicanum). J Neurocytol 5:33–41
Knowlton VY (1967) Correlation of the development of membranous and bony labyrinth, acoustic ganglia, nerves and brain centers in the chick embryo. J Morphol 121:179–207
Mbiene J-P, Favre D, Sans A (1984) The pattern of ciliary development in fetal mouse vestibular receptors. A qualitative and quantitative SEM study. Anat Embryol 170:229–238
Mbiene J-P, Sans A (1986) The differentiation and maturation of the sensory hairs in the foetal and postnatal vestibular receptors in the mouse. A scanning electron microscopy study. J Comp Neurol 254:271–278
Mirsky R, Jessen KR, Schachner M, Goridis C (1986) Distribution of the adhesion molecules N-CAM and L1 on peripheral neurons and glia in adult rats. J Neurocytol 15:693–714
Nordemar H (1983) Embryogenesis of inner ear. II. The late differentiation of the mammalian crista ampullaris in vivo and in vitro. Acta Otolaryngol (Stockh) 96:1–8
Orr MF (1968) Histogenesis of sensory epithelium in reaggregates of dissociated embryonic chick otocysts. Dev Biol 17:39–54
Privat A (1974) A possible mechanism for the resorption of attachment plates in the growing rat brain. Brain Res 69: 125–129
Pujol R, Lavigne-Rebillard M (1985) Early stages of innervation and sensory cell differentiation in the human fetal organ of Corti. Acta Otolaryngol (Stockh) Suppl 423:43–50
Pujol R, Sans A (1986) Synaptogenesis in the cochlear and vestibular receptor. In: Aslin R (ed) Advances in Neural and Behavioral Development, vol 2. Ablex Press, Norwood New Jersey, pp 1–18
Sans A, Chat M (1982) Analysis of temporal and spatial patterns of rat vestibular hair cell differentiation by tritiated thymidine radioautography. J Comp Neurol 206:1–8
Sans A, Dechesne C (1985) Early development of vestibular receptors in human embryos: An electron microscopic study. Acta Otolaryngol (Stockh) Suppl 423:51–58
Sans A, Dechesne C (1987) Afferent nerve ending development and synaptogenesis in the vestibular epithelium of human fetuses. Hearing Res 28:65–72
Sher AE (1971) The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol (Stockh), Suppl 285:1–77
Sobkowicz HM, Rose JE, Scott GL, Slapnick SM (1982) Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci 2:942–957
Sobkowicz HM, Rose JE, Scott GL, Levenick CV (1986) Distribution of synaptic ribbons in the developing organ of Corti. J Neurocytol 15:693–714
Thornhill RA (1972) The development of the labyrinth of the lamprey (Lampreta fluviatilis Linn. 1758). Proc R Soc, London B181:175–198
Van de Water TR (1976) Effects of removal of the stato-acoustic ganglion complex upon the growing otocyst. Ann Otol Rhinol Laryngol Suppl 33:85 1–32
Van de Water TR, Anniko M, Nordemar H, Wersäll J (1977) Embryonic development of the sensory cells in the macula utriculi of mouse. Colloques INSERM, Symp 68 (Inner Ear Biology), pp 25–36
Van de Water TR, Ruben RJ (1984) Neurotrophic interactions during in vitro development of the inner ear. Ann Otorhinolaryngol 93:558–564
Van de Water TR (1986) Determinants of neuron-sensory receptor cell interaction during development of the inner ear. Hearing Res 22:265–277
Vaughn JE, Henrikson CK, Wood JG (1976) Surface specializations of neurites in embryonic mouse spinal cord. Brain Res 110:431–445
Vaughn JE, Sims TJ (1978) Axonal growth cones and developing axonal collaterals form synaptic junctions in embryonic mouse spinal cord. J Neurocytol 7:337–363
Whitehead MC, Morest DK (1985) The growth of cochlear fibers and the formation of their synaptic endings in the avian inner ears: a study with the electron microscope. Neuroscience 14:277–300
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mbiene, J.P., Favre, D. & Sans, A. Early innervation and differentiation of hair cells in the vestibular epithelia of mouse embryos: SEM and TEM study. Anat Embryol 177, 331–340 (1988). https://doi.org/10.1007/BF00315841
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315841