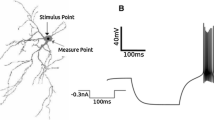
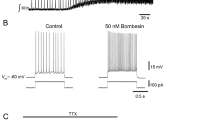
Summary
1. Spikes of single neurons were recorded extracellularly in the cat prepositus hypoglossi nucleus and the underlying reticular formation and were identified as type II neurons by horizontal rotation. Among these neurons, those activated by contralateral vestibular nerve stimulation with short latencies (1.5–3.0 ms) were selected for further study. 2. A class of these identified neurons was antidromically activated from the contralateral excitatory burst neuron (EBN) area immediately rostral to the abducens nucleus. Systematic tracking for antidromic stimulation revealed a wide distribution of effective spots in and near the EBN area, with varied latencies and thresholds, suggesting terminal branching in that area. The same neurons were also antidromically activated from the contralateral inhibitory burst neuron (IBN) area, the region near the midline, and the nucleus reticularis tegmenti pontis. 3. These neurons exhibited a characteristic firing pattern related to nystagmus: with contralateral rotation the firing rate gradually increased during the slow phase (type II response) and further steeply increased in a burst fashion before and during the contraversive quick phase. Since the time of occurrence of burst activity in these neurons was similar to that of contralateral ENBs and IBNs that received their axonal projection, it is suggested that they send excitatory input to burst neurons, and can thus be called burster-driving neurons (BDNs). 4. Intracellular study revealed that stimulation of the BDN area produced monosynaptic EPSPs in contralateral EBNs. The monosynaptic connection of BDNs with EBNs was confirmed by detecting unitary extracellular synaptic currents of EBNs with the spike-triggered averaging technique. 5. In contrast to BDNs, another class of nystagmus-related type II neurons in the prepositus hypoglossi and medullary reticular formation showed a discharge pattern similar to that of abducens motoneurons on the same side. None of them was antidromically activated from the contralateral pontine reticular formation including the EBN area. Some neurons responded anti-dromically to stimulation of the ipsilateral dorsomedial pontine reticular formation. 6. In conclusion, the input from the horizontal canal during rotation reaches the contralateral prepositus hypoglossi nucleus and the underlying reticular formation through the vestibular nuclei, and a class of neurons in these structures (BDNs) responds to the canal input in a burst fashion following a tonic type II activity. The axons of BDNs cross the midline and monosynaptically excite EBNs on the side of the canal stimulated. The burst activity of BDNs at the quick phase is suggested to contribute to generation of spike burst of EBNs and IBNs.
Similar content being viewed by others
References
Alley K, Baker R, Simpson JI (1975) Afferents to the vestibulocerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res 98: 582–589
Baker R, Berthoz A (1975) Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res 86: 121–127
Baker R, Gresty M, Berthoz A (1975) Neuronal activity in the prepositus correlated with vertical and horizontal eye movement in the cat. Brain Res 101: 366–371
Baker R, Mano N, Shimazu H (1969) Postsynaptic potentials in abducens motoneurons induced by vestibular stimulation. Brain Res 15: 577–580
Batini R, Buisseret-Delmas C, Corvisier J, Hardy O, Jassik-Gerschenfeld D (1978) Brain stem nuclei giving fibers to lobules VI and VII of the cerebellar vermis. Brain Res 153: 241–261
Brodal A (1952) Experimental demonstration of cerebellar connexions from the peri-hypoglossal nuclei (nucleus intercalatus, nucleus praepositus hypoglossi, and nucleus of Roller) in the cat. J Anat 86: 110–129
Cohen B, Henn V (1972) Unit activity in the pontine reticular formation associated with eye movement. Brain Res 46: 403–410
Crandall WF, Keller EL (1985) Visual and oculomotor signals in nucleus reticularis tegmenti pontis in alert monkey. J Neurophysiol 54: 1326–1345
Curthoys IS, Markham CH, Furuya N (1984) Direct projection of pause neurons to nystagmus-related excitatory burst neurons in the cat pontine reticular formation. Exp Neurol 83: 414–422
Evinger C, Kaneko CRS, Fuchs AF (1982) The activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J Neurophysiol 47: 827–844
Evinger C, McCrea RA, Baker R (1980) Intracellular injection of HRP into omnipause neurons in the alert cat. Soc Neurosci Abstr 6: 16
Fuchs AF, Luschei ES (1970) Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol 33: 382–392
Fukushima Y, Igusa Y, Yoshida K (1977) Characteristics of responses of medial brain stem neurons to horizontal head angular acceleration and electrical stimulation of the labyrinth in the cat. Brain Res 120: 564–570
Furuya N, Markham CH (1982) Direct inhibitory synaptic linkage of pause neurons with burst inhibitory neurons. Brain Res 245: 139–143
Henn V, Cohen B (1976) Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res 108: 307–325
Hikosaka O, Igusa Y (1980) Axonal projection of the prepositus hypoglossi and reticular neurons in the brain stem of the cat. Exp Brain Res 39: 441–451
Hikosaka O, Igusa Y, Imai H (1978a) Firing pattern of prepositus hypoglossi and adjacent reticular neurons related to vestibular nystagmus in the cat. Brain Res 144: 395–403
Hikosaka O, Igusa Y, Nakao S, Shimazu H (1978b) Direct inhibitory synaptic linkage of pontomedullary reticular burst neurons with abducens motoneurons in the cat. Exp Brain Res 33: 337–352
Hikosaka O, Kawakami T (1977) Inhibitory reticular neurons related to the quick phase of vestibular nystagmus — their location and projection. Exp Brain Res 27: 377–396
Hikosaka O, Maeda M, Nakao S, Shimazu H, Shinoda Y (1977) Presynaptic impulses in the abducens nucleus and their relation to postsynaptic potentials in motoneurons during vestibular nystagmus. Exp Brain Res 27: 355–376
Igusa Y, Sasaki S, Shimazu H (1980) Excitatory premotor burst neurons in the cat pontine reticular formation related to the quick phase of vestibular nystagmus. Brain Res 182: 451–456
Ishizuka N, Mannen H, Sasaki S, Shimazu H (1980) Axonal branches and terminations in the cat abducens nucleus of secondary vestibular neurons in the horizontal canal system. Neurosci Lett 16: 143–148
Jankowska E, Roberts WJ (1972a) An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol (Lond) 222: 597–622
Jankowska E, Roberts WJ (1972b) Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol (Lond) 222: 623–642
Kase M, Miller DC, Noda H (1980) Discharges of Purkinje cells and mossy fibers in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J Physiol (Lond) 300: 539–555
Keller EL (1974) Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol 37: 316–332
Keller EL, Crandall WF (1981) Neuronal activity in the nucleus reticularis tegmenti pontis in the monkey related to eye movements and visual stimulation. In: Cohen B (ed) Vestibular and oculomotor physiology. Ann NY Acad Sci 374: 249–261
Kotchabhadki N, Hoddevik GH, Walberg F (1978) Cerebellar afferent projections from the perihypoglossal nuclei: an experimental study with the method of retrograde axonal transport of horseradish peroxidase. Exp Brain Res 31: 13–29
Langer TP, Kaneko CRS (1983) Efferent projections of the cat oculomotor reticular omnipause neuron region: an autoradiographic study. J Comp Neurol 217: 288–306
Lisberger SG, Fuchs AF (1978) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. II. Mossy fiber firing patterns during horizontal head rotation and eye movement. J Neurophysiol 41: 764–777
Llinás R, Wolfe JW (1977) Functional linkage between the electrical activity in the vermal cerebellar cortex and saccadic eye movements. Exp Brain Res 29: 1–14
Lopez-Barneo J, Darlot C, Berthoz A, Baker R (1982) Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J Neurophysiol 47: 329–352
Luschei ES, Fuchs AF (1972) Activity of brain stem neurons during eye movement of alert monkey. J Neurophysiol 35: 445–461
Maeda M, Shimazu H, Shinoda Y (1972) Nature of synaptic events in cat abducens motoneurons at slow and quick phase of vestibular nystagmus. J Neurophysiol 35: 279–296
Markham CH, Nakao S, Curthoys IS (1981) Cat medial pontine neurons in vestibular nystagmus. In: Cohen B (ed) Vestibular and oculomotor physiology. Ann NY Acad Sci 374: 189–209
McCrea RA, Baker R (1985) Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol 237: 377–407
McCrea RA, Yoshida K, Berthoz A, Baker R (1980) Eye movement related activity and morphology of second order vestibular neurons terminating in the cat abducens nucleus. Exp Brain Res 40: 468–473
McCrea RA, Yoshida K, Evinger C, Berthoz A (1981) The location, axonal arborization and termination sites of eye-movement-related secondary vestibular neurons demonstrated by intra-axonal HRP injection in the alert cat. In: Fuchs AF, Becker W (eds) Progress in oculomotor research. Elsevier, New York, pp 379–386
McElligott JG, Keller EL (1982) Neuronal discharge in the posterior cerebellum: its relationship to saccadic eye movement generation. In: Lennerstrand G, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon, Oxford, pp 453–461
Miles FA, Fuller JH, Braitman DJ, Dow BM (1980) Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological obervations in flocculus of normal monkeys. J Neurophysiol 43: 1437–1476
Nakao S, Curthoys IS, Markham CH (1980) Direct inhibitory projection of pause neurons to nystagmus-related pontomedullary reticular burst neurons in the cat. Exp Brain Res 40: 283–293
Nakao S, Sasaki S, Schor RH, Shimazu H (1982) Functional organization of premotor neurons in the cat medial vestibular nucleus related to slow and fast phases of nystagmus. Exp Brain Res 45: 371–385
Noda H, Suzuki DA (1979) The role of the flocculus of the monkey in saccadic eye movements. J Physiol (Lond) 294: 317–334
Ohki Y, Shimazu H, Suzuki I (1985) A pathway transmitting vestibular input to excitatory burst neurons related to horizontal nystagmus. Neurosci Res, Suppl 1: S57
Ohki Y, Shimazu H, Suzuki I (1988) Functional organization of the horizontal vestibulo-oculomotor system in the cat brain stem. In: Hwang JC, Daunton NG, Wilson VJ (eds) Basic and applied aspects of vestibular function. Hong Kong University Press, Hong Kong (in press)
Rapoport S, Susswein A, Uchino Y, Wilson VJ (1977) Synaptic actions of individual vestibular neurones on cat neck motoneurones. J Physiol (Lond) 272: 367–382
Robinson DA (1970) Oculomotor unit behavior in the monkey. J Neurophysiol 33: 393–404
Robinson DA (1975) Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon, New York, pp 337–378
Sasaki S, Shimazu H (1981) Reticulovestibular organization participating in generation of horizontal fast eye movement. In: Cohen B (ed) Vestibular and oculomotor physiology. Ann NY Acad Sci 374: 130–143
Strassman A, Evinger C, McCrea RA, Baker RG, Highstein SM (1987) Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp Brain Res 67: 436–440
Strassman A, Highstein SM, McCrea RA (1986a) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249: 337–357
Strassman A, Highstein SM, McCrea RA (1986b) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249: 358–380
Thomas RC, Wilson VJ (1965) Precise localization of Renshaw cells with a new marking technique. Nature 206: 211–213
Torvik A, Brodal A (1954) The cerebellar projection of the perihypoglossal nuclei (nucleus intercalatus, nucleus praepositus hypoglossi and nucleus of Roller) in the cat. J Neuropathol 13: 515–527
Waespe W, Henn V (1981) Visual-vestibular interaction in the flocculus of the alert monkey. II. Purkinje cell activity. Exp Brain Res 43: 349–360
Yoshida K, McCrea RA, Berthoz A, Vidal PP (1982) Morphological and physiological characteristics of inhibitory burst neurons controlling horizontal rapid eye movements in the alert cat. J Neurophysiol 48: 761–784
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohki, Y., Shimazu, H. & Suzuki, I. Excitatory input to burst neurons from the labyrinth and its mediating pathway in the cat: location and functional characteristics of burster-driving neurons. Exp Brain Res 72, 457–472 (1988). https://doi.org/10.1007/BF00250591
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00250591