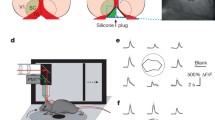
Summary
Single unit recordings in the nucleus of the basal optic root (nBOR) of the accessory optic system in chickens suggest that it has a role in vertical stabilizing eye movements. Cells have unusually large receptive fields and never respond to small stationary stimuli. They respond best to large richly patterned stimuli moving slowly (2–4 °/s) in vertical directions. Cells responsive to upward movement tend to be located in the dorsal portion of nBOR, which projects to motor areas producing upward eye movement, whereas cells responsive to downward movement tend to be located in the ventral portion of nBOR, which projects to motor areas producing downward eye movement; this suggests that these synapses onto oculomotor neurons are excitatory.
In many nBOR units, the preferred and null directions are not opposite to each other. These directional asymmetries seem to be correlated with other properties of the units in a manner that supports the idea that the accessory optic system is arranged according to a vestibular coordinate system. This finding complements the abundant anatomical and physiological evidence linking the accessory optic system to the vestibular system.
Similar content being viewed by others
References
Baarsma EA, Collewijn H (1974) Vestibulo-ocular and optokinetic reactions to rotation and their interaction in the rabbit. J Physiol 238: 603–625
Barlow HB, Levick WR (1965) The mechanism of directionally selective units in rabbit's retina. J Physiol 178: 477–504
Barmack NH, Hess DT (1980a) Multiple-unit activity evoked in dorsal cap of inferior olive of the rabbit by visual stimulation. J Neurophysiol 43: 151–164
Barmack NH, Hess DT (1980b) Eye movements evoked by microstimulation of dorsal cap of inferior olive in the rabbit. J. Neurophysiol 43: 165–181
Barmack NH, Simpson JI (1980) Effects of microlesions of dorsal cap of inferior olive of rabbits on optokinetic and vestibulo-ocular reflexes. J Neurophysiol 43: 182–206
Batini, C, Ito M, Kado RT, Jastreboff PJ, Miyashita Y (1979) Interaction between the horizontal vestibulo-ocular reflex and optokinetic response in rabbits. Exp Brain Res 37: 1–16
Brauth SE, Karten HJ (1977) Direct accessory optic projections to the vestibulo-cerebellum; a possible channel for oculomotor control systems. Exp Brain Res 28: 73–84
Brecha N, Karten HJ (1979) Accessory optic projections upon oculomotor nuclei and vestibulocerebellum. Science 203: 913–916
Brecha N, Karten HJ, Hunt SP (1980) Projections of the nucleus of the basal optic root in the pigeon: An autoradiographic and horseradish peroxidase study. J Comp Neurol 189: 615–670
Burns S, Wallman J (1979) Neurons in the nucleus of the basal optic root (accessory optic system) of birds respond preferentially to vertical stimulus movement. Neurosci Abst 5: 779
Carpenter RHS (1977) Movements of the eyes. Pion, London
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic afternystagmus. J Physiol 270: 321–344
Collewijn H (1972) An analog model of the rabbit's optokinetic system. Brain Res 36: 71–88
Crandall JE, Heaton MB, Brownell WE (1977) Tectal projection of displaced ganglion cells in avian retina. Invest Ophthalmol 16: 774–776
Daniels PD, Hassul M, Kimm J (1978) Dynamic analysis of the vestibulo-ocular reflex in the normal and flocculectomized chinchilla. Exp Neurol 58: 32–45
Famiglietti EV, Kaneko A, Tachibana M (1977) Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science 198: 1267–1269
Finger T, Karten HJ (1978) The accessory optic system in teleosts. Brain Res 153: 144–149
Fite KV, Reiner A, Hunt SP (1979) Optokinetic nystagmus and the accessory optic system of pigeon and turtle. Brain Behav Evol 16: 192–202
Haddad GM, Demer JL, Robinson DA (1980) The effect of lesions of the dorsol cap of the inferior olive on the vestibulo-ocular and optokinetic systems of the cat. Brain Res 185: 265–275
Heaton MB, Alvarez IM, Crandall JE (1979) The displaced ganglion cell in the avian retina: Developmental and comparative considerations. Anat Embryol 155: 161–178
Ito M, Nisimaru N, Yamamoto M (1977) Specific patterns of neuronal connexions involved in the control of the rabbit's vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol 265: 833–854
Ito M, Shiida T, Yagi N, Yamamoto M (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res 65: 170–174
Karten HJ (1979) Visual lemniscal pathways in birds. In: Granda AM, Maxwell JH (eds) Neural mechanisms of behavior in the pigeon. Plenum Press, New York London, pp 409–430
Karten HJ, Fite KV, Brecha N (1977) Specific projection of displaced retinal ganglion cells upon the accessory optic system in the pigeon (Columba livia). Proc Natl Acad Sci USA 74: 1753–1756
Kimm J, Winfield JA, Hendrickson AE (1979) Visual-vestibular interactions and the role of the flocculus in the vestibulo-ocular reflex. In: Granit R, Pomeiano O (eds) Reflex control of posture and movement. Progress in brain research, vol 50, Elsevier/North Holland, Amsterdam New York, pp 703–713
Lázár G (1973) Role of the accessory optic system in the optokinetic nystagmus of the frog. Brain Behav Evol 5: 443–460
Maekawa K, Simpson JI (1972) Climbing fiber activation of Purkinje cells in the flocculus by impulses transferred through the visual pathway. Brain Res 39: 245–251
Maekawa K, Simpson JI (1973) Climbing fiber response evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol 36: 649–666
Maekawa K, Takeda T (1977) Afferent pathways from the visual system to the cerebellar flocculus of the rabbit. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons. Elsevier/North Holland, Amsterdam New York, pp 187–196
McKenna O, Wallman J (1981) Identification of avian brain regions responsive to retinal slip using 2-deoxyglucose. Brain Res (in press)
Miles FA (1972) Centrifugal control of the avian retina. I. Receptive field properties of retinal ganglion cells. Brain Res 48: 65–92
Montgomery N, Fite K, Bengston L (1979) The anurian accessory optic system: Neuroanatomical analysis. Neurosci Abst 5: 798
Morgan B, Frost BJ (1979) Single unit and behavioral assessment of ecto-mamillary nucleus function. Neurosci Abst 5: 799
Morgan B, Frost BJ (1981) Visual response characteristics of neurons in nucleus of basal optic root of pigeons. Exp Brain Res 42: 181–188
Nelson R, Famiglietti EV, Kolb H (1978) Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol 41: 472–483
Oyster CW (1968) The analysis of image motion by the rabbit retina. J Physiol 199: 613–635
Oyster CW, Simpson JI, Takahashi ES, Soodak RE (1980) Retinal ganglion cells projecting to the rabbit accessory optic system. J Comp Neurol 190: 49–61
Pearlman AL, Hughes CP (1976) Functional role of retinal ganglion cell receptive fields. I. Analysis of retinal ganglion cell receptive fields. J. Comp Neurol 166: 111–122
Pease PL (1973) Eye movements in systemically paralyzed macaque monkeys. Vision Res 13: 483–487
Raphan Th, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248
Reiner A, Brecha N, Karten HJ (1979) A specific projection of retinal displaced ganglion cells to the nucleus of the basal optic root in the chicken. Neuroscience 4: 1679–1688
Reiner A, Karten HJ (1978) A bisynaptic retinocerebellar pathway in the turtle. Brain Res 151: 163–169
Repérant J (1973) Nouvelles donnees sur les projections visuelles chez le pigeon (Columba livia). J Hirnforsch 14: 151–187
Robinson DA (1976) Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol 39: 954–969
Simpson JI, Alley KE (1974) Visual climbing fiber input to rabbit vestibulo-cerebellum: A source of direction-specific information. Brain Res 82: 302–308
Simpson JI, Hess R (1977) Complex and simple visual messages in the flocculus. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons. Elsevier/North Holland, Amsterdam New York, pp 351–360
Simpson JI, Soodak RE, Hess R (1979) The accessory optic system and its relation to the vestibulo-cerebellum. In: Granit R, Pomeiano O (eds) Reflex control of posture and movement. Progress in brain research, vol. 50. Elsevier/North Holland, Amsterdam New York, pp 715–724
Suzuki J, Cohen B, Bender MB (1964) Compensatory eye movements induced by vertical semicircular canal stimulation. Exp Neurol 9: 137–160
Waespe W, Henn V (1977) Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res 27: 523–538
Walley RE (1967) Receptive fields in the accessory optic system of the rabbit. Exp Neurol 17: 27–43
Westheimer G, Blair SM (1974) Unit activity in accessory optic system in alert monkey. Invest Ophthalmol 13: 533–534
Winfield JA, Hendrickson A, Kimm J (1978) Anatomical evidence that the medial terminal nucleus of the accessory optic tract in mammals provides a visual mossy fiber input to the flocculus. Brain Res 151: 175–182
Wyatt HJ, Daw NW (1975) Directionally sensitive ganglion cells in the rabbit retina: Specificity for stimulus direction, size, and speed. J Neurophysiol 38: 613–626
Zar JH (1974) Biostatistical analysis. Prentice-Hall, Englewood Cliffs, NJ
Author information
Authors and Affiliations
Additional information
This work was supported by a research grant from the National Eye Institute, EY02937
Rights and permissions
About this article
Cite this article
Burns, S., Wallman, J. Relation of single unit properties to the oculomotor function of the nucleus of the basal optic root (accessory optic system) in chickens. Exp Brain Res 42, 171–180 (1981). https://doi.org/10.1007/BF00236903
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00236903