Summary
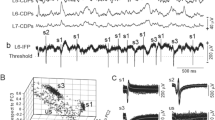
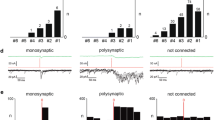
Cat dorsal horn was searched for all detectable units that responded to peripheral C fibre input. Fifty-seven such units were examined in detail. They were located in two main areas. One group was in the superficial laminae 1, 2, and possibly dorsal 3 (n = 29), and the other group was much deeper in laminae 5 and 6 (n = 24). Only four units were situated in the region of lamina 4.
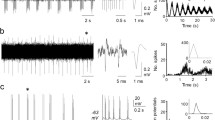
Differences were found in the responses to C fibre stimulation of these two groups, both in the optimum stimulus and in the timing of responses to repeated stimulation. Superficial units often did not respond to C fibre stimulation unless a train of two or more stimuli (10 ms apart) were applied, but when responses did occur they were usually very even and regular, with precise onset latencies on repeated stimulation. Deep units tended to need only one peripheral C fibre stimulus for excitation, but the responses were irregular with latencies fluctuating with each stimulus. Some superficial and deep units showed a steady increase in latency of the late C response on repeated stimulation. Increases of up to 80 ms after 30 s of stimulation at 1 Hz were observed.
The results are discussed in terms of the neuronal connections in the dorsal horn.
Similar content being viewed by others
References
Abrahams VC (1974) On the induction of prolonged change in the functional state of the spinal cord. In: Bonica JJ (ed) Advances in neurology, vol 4. Raven Press, New York
Bessou P, Perl ER (1969) Responses of cutaneous sensory units with unmyelinated fibres to noxious stimuli. J Neurophysiol 32: 1025–1043
Brown AG, Hamann WC, Martin HF (1975) Effects of activity in non-myelinated afferent fibres on the spinocervical tract. Brain Res 98: 243–259
Brown AG, House CR, Rose PK, Snow PJ (1976) The morphology of spinocervical tract neurones in the cat. J Physiol (Lond) 260: 719–738
Christensen BN, Perl ER (1970) Spinal neurons specifically excited by noxious and thermal stimuli. Marginal zone of the dorsal horn. J Neurophysiol 33: 293–307
Dubuisson D, Fitzgerald M, Wall PD (1979) Ameboid receptive fields of cells in laminae 1, 2 and 3. Brain Res 177: 376–378
Fitzgerald M (1980) An electrophysiological study of the afferent input to substantia gelatinosa of the cat. (submit.)
Gobel S, Binck JM (1977) Degenerative changes in primary trigeminal axons following tooth pulp extirpation. Brain Res 132: 347–354
Gregor M, Zimmerman M (1972) Characteristics of spinal neurons responding to cutaneous myelinated and unmyelinated fibres. J Physiol (Lond) 221: 555–576
Handwerker HO, Iggo A, Zimmerman M (1975) Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimulation. Pain 1: 147–165
Hentall ID, Fields HL (1979) Segmental and descending influences on the thresholds of single intraspinal C fibres. J Neurophysiol 42: 1527–1537
Iggo A, Ogawa H, Cervero F (1976) Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain 2: 5–24
Kerr FWL (1975) Neuroanatomical substrates of nociception in the spinal cord. Pain 1: 325–356
Kumuzawa T, Perl ER (1978) Excitation of marginal and substantia gelatinosa neurons in primate spinal cord. Indications of their place in dorsal horn functional organization. J Comp Neurol 177: 417–434
Lamotte C (1977) Distribution of the tract of Lissauer and dorsal root fibres in primate spinal cord. J Comp Neurol 172: 529–562
Light AR, Perl ER (1979) Re-examination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibres. J Comp Neurol 186: 117–131
Light AR, Trevino DL, Perl, ER (1979) Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol 186: 151–172
Mendell LM (1966) Physiological properties of unmyelinated fibre projection to the spinal cord. Exp Neurol 16: 316–322
Merrill EG (1974) Iron-plated tungsten microelectrodes for tip marking. J Physiol (Lond) 241: 68–69P
Merrill EG, Ainsworth A (1972) Glass-coated platinum plated tungsten microelectrodes. Med Biol Eng 10: 662–672
Ochoa J, Mair WGP (1969) The normal sural nerve in man. I. Ultrastructure and numbers of fibres and cells. Acta Neuropathol (Berl) 13: 197
Proshansky E, Egger MD (1977) Dendritic spread of dorsal horn neurons in cats. Exp Brain Res 28: 153–166
Rethelyi M, Szentágothai J (1973) Distribution and connections of afferent fibres in the spinal cord. In: Iggo A (ed) Handbook of sensory physiology, vol 2. Springer, Berlin Heidelberg New York
Rexed B (1952) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100: 297–379
Snedecor GW, Cochran WG (1973) Statistical methods. Iowa State University Press, pp 135–195
Szentágothai J (1964) Neuronal and synaptic arrangement in the substantia gelatinosa Rolandi. J Comp Neurol 122: 219–240
Wagman IH, Price DD (1969) Responses of dorsal horn cells of M. mulatta to cutaneous and sural nerve A and C fibre stimulation. J Neurophysiol 32: 803–817
Wall PD (1959) Repetitive discharge of neurons. J Neurophysiol 22: 305–320
Wall PD (1967) The laminar organization of the dorsal horn and the effects of descending impulses. J Physiol 188: 403–423
Wall PD, Hillman P (1969) Inhibitory and excitatory factors controlling lamina 5 cells. Exp Brain Res 9: 284–306
Wall PD, Merrill EG, Yaksh TL (1979) Responses of single units in laminae 2 and 3 of cat spinal cord. Brain Res 160: 245–260
Zimmerman M (1978) Afferent control of pain at the spinal segmental level. Neurosci Lett [Suppl] 1: 437
Author information
Authors and Affiliations
Additional information
The work was supported by the Medical Research Council and the National Institutes of Health
M. Fitzgerald is a Medical Research Council Training Fellow
Rights and permissions
About this article
Cite this article
Fitzgerald, M., Wall, P.D. The laminar organization of dorsal horn cells responding to peripheral C fibre stimulation. Exp Brain Res 41, 36–44 (1980). https://doi.org/10.1007/BF00236677
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00236677