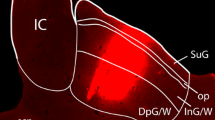
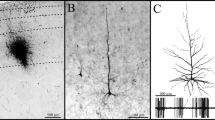
Abstract
Neurons in layer III of the medial entorhinal area (MEA) in the rat are extremely vulnerable to local injections of amino-oxyacetic acid and to exprimentally induced limbic seizures. A comparable specific pathology has been noted in surgical specimens from patients with temporal lobe epilepsy. Efforts to understand this preferential neuronal vulnerability led us to study the neural input to this layer in the rat. Iontophoretic injection of the retrograde tracer fast blue, aimed at layer III of the MEA, resulted in retrogradely labeled neurons in the presubiculum in all the injected hemispheres. The nucleus reuniens thalami, the anteromedial thalamic nucleus, the ventral portion of the claustrum (endopiriform nucleus), the dorsomedial parts of the anteroventral thalamic nucleus, and the septum-diagonal band complex were labeled less frequently. In only one experiment, retrogradely labeled neurons were observed in the ventrolateral hypothalamus and in the brainstem nucleus raphe dorsalis. Since projections from claustrum to the entorhinal cortex has not been studied in the rat with modern sensitive anterograde tracing techniques, iontophoretic injections of the anterograde tracer Phaseolus vulgaris-leucoagglutinin were placed into the ventral portion of the claustrum. Anterogradely labeled fibers in the entorhinal area proved not to be confined to the MEA, since a prominent projection distributed to the lateral entorhinal area as well. In both areas, the densest terminal labeling was present in layers IV–VI, whereas layer III appeared to be only sparsely labeled. The present data indicate that of all potential afferents only those from the presubiculum distribute preferentially to layer III of the MEA. This, in turn, suggests a potentially important role of the presubiculum in the seizure-related degeneration of neurons in layer III of the MEA.
Similar content being viewed by others
References
Alonso A, Köhler C (1984) A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J Comp Neurol 225:327–342
Bartesaghi R, Gessi T, Migliore M (1995) Input-output relations in the entorhinal-hippocampal-entorhinal loop: entorhinal cortex and dentate gyrus. Hippocampus 5:440–451
Beckstead RM (1978) Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res 152:249–264
Bekenstein JW, Lothman EW (1993) Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science 259:97–100
Biziere K, Coyle JT (1978) Influence of cortico-striatal afferents on striatal kainic acid neurotoxicity. Neurosci Lett 8:303–310
Blackstad TW (1956) Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol 105:417–537
Caballero-Bleda M, Witter MP (1993) Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: an anterograde tracing study in the rat. J Comp Neurol 328:115–129
Cabellero-Bleda M, Witter MP (1994) Projections from the presubiculum and the parasubiculum to morphologically characterized entorhinal-hippocampal projection neurons in the rat. Exp Brain Res 101:93–108
Canteras NS, Simerly RB, Swanson LW (1994) Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:41–79
Clifford DB, Olney JW, Benz AM, Fuller TA, Zoruski CF (1990) Ketamine, phencyclidine, and MK-801 protect against kainic acid-induced seizure-related brain damage. Epilepsia 31:382–390
Collins RC, Tearse RG, Lothman EW (1983) Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res 280:25–40
Colonnier M (1968) Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res 9:268–287
Cross AJ, Jones JA, Baldwin HA, Green AR (1991) Neuroprotective activity of chlormethiazole following transient forebrain ischaemia in the gerbil. Br J Pharmacol 104:406–411
Du F, Schwarcz R (1992) Aminooxyacetic acid causes selective neuronal loss in layer III of the rat medial entorhinal cortex. Neurosci Lett 147:185–188
Du F, Whetsell WO Jr, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R (1993) Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res 16:223–233
Du F, Eid T, Lothman EW, Köhler C, Schwarcz R (1995) Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci 15:8301–8313
During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizures in the conscious human brain. Lancet 341:1607–1611
Eid T, Du F, Schwarcz R (1995) Differential neuronal vulnerability to aminooxyacetic acid and quinolinic acid in the rat parahippocampal region. Neuroscience 68:645–656
Gastaut H (1956) Colloque sur les problemes d'anatomie normale et pathologique poses par les decharges epileptiques. Acta Med Belg 5–66
Gaykema RPA, Luiten PGM, Nyakas C, Traber J (1990) Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol 293:103–124
Haug FMS (1976) Sulphide silver pattern and cytoarchitectonics of parahippocampal areas in the rat. Special reference to the subdivision of area entorhinalis (area 28) and its demarcation from the pyriform cortex. Adv Anat Embryol Cell Biol 52:1–73
Haeften T van, Wouterlood FG, Jorritsma-Byham B, Witter MP (1995) Light-and electron-microscopic demonstration of a presubicular GABAergic projection to the medial entorhinal cortex of the rat (abstract). Eur J Neurosci [Suppl] 8:153
Johansen FF, Diemer NH (1991) Enhancement of GABA neurotransmission after cerebral ischemia in the rat reduces loss of hippocampal CA1 pyramidal cells. Acta Neurol Scand 84:1–6
Jones RSG (1993) Entorhinal-hippocampal connections: a speculative view of their function. Trends Neurosci 16:58–64
Köhler C (1985) Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol 236:504–522
Köhler C (1986) Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol 246:149–169
Köhler C, Schwarcz R, Fuxe K (1978) Perforant path transections protect hippocampal granule cells from kainate lesion. Neurosci Lett 10:241–246
Krettek JE, Price JL (1977) Projections from the amyglaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol 172:723–752
McGeer EG, McGeer PL, Singh K (1978) Kainate-induced degeneration of neostriatal neurons: dependency upon corticostriatal tract. Brain Res 139:381–383
Meldrum BS (1995) Neurotransmission in epilepsy. Epilepsia 36:S30-S35
Nadler JV, Cuthbertson GJ (1980) Kainic acid neurotoxicity toard hippocampal formation: dependence on specific excitatory pathways. Brain Res 195:47–56
Olney JW, Collins RC, Sloviter RS (1986) Excitotoxic mechanisms of epileptic brain damage. Adv Neurol 44:857–877
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic, San Diego, CA
Ramón y Cajal S (1911) Histologie du systeme nerveux de l'homme et des vertebrés. Maloine, Paris
Raviola G, Raviola E (1967) Light and electron-microscopic observations on the inner plexiform layer of the rabbit retina. Am J Anat 120:403–426
Risold PY, Canteras NS, Swanson LW (1994) Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:1–40
Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S (1989) Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg 70:667–675
Ruth RE, Collier TJ, Routtenberg A (1982) Topography between the entorhinal cortex and the dentate septotemporal axis in rats. I. Medial and intermediate entorhinal projecting cells. J Comp Neurol 209:69–78
Ruth RE, Collier TJ, Routtenberg A (1988) Topographical relationship between the entorhinal cortex and the septotemporal axis of the dentate gyrus in rats. II. Cells projecting from lateral entorhinal subdivisions. J Comp Neurol 270:506–516
Segal M (1977) Afferents to the entorhinal cortex of the rat studied by the method of retrograde transport of horseradish peroxidase. Exp Neurol 57:750–765
Sloviter RS (1991) Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1:41–66
Spencer SS, Spencer DD (1994) Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia 35:721–727
Steward O (1976) Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167:285–314
Steward O, Scoville SA (1976) Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol 169:347–370
Swanson LW, Köhler C, Björklund A (1987) The limbic region. I. The septohippocampal system. In: Björklund A, Hökfelt T, Swanson LW (eds) (Handbook of chemical neuroanatomy, vol 5) Integrated systems of the CNS, part I. Elsevier, Amsterdam, pp 125–227
]Uchizono K (1965) Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207:642–643
Vertes RP (1991) A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313:643–668
Vertes RP (1992) PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol 326:595–622
Witter MP, Room P, Groenewegen HJ, Lohman AHM (1986) Reciprocal connections of the insular and piriform claustrum with limbic cortex: an anatomical study in the cat. Neuroscience 24:519–539
Witter MP, Griffioen AW, Jorritsma-Byham B, Krijnen JLM (1988) Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci Lett 85:193–198
Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM (1989a) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Progr Neurobiol 33:161–253
Witter MP, Van Hoesen GW, Amaral DG (1989b) Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9:216–228
Witter MP, Eid T, Schwarcz R (1994) Anatomical analysis of afferent connections of layer III of the medial entorhinal cortex in the rat. Soc Neurosci Abstr 20:151. 15
Wouterlood FG, Saldana E, Witter MP (1990) Projection from the nucleus reiniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 296:179–203
Wouterlood FG, Haeften T van, Witter MP (1995) Demonstration of a presubicular GABAergic projection to the medial entorhinal cortex of the rat. Soc Neurosci Abstr 21:428
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eid, T., Jorritsma-Byham, B., Schwarcz, R. et al. Afferents to the seizure-sensitive neurons in layer III of the medial entorhinal area: a tracing study in the rat. Exp Brain Res 109, 209–218 (1996). https://doi.org/10.1007/BF00231782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231782