Summary
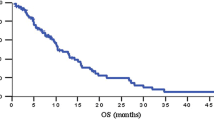
Esorubicin (4′ deoxydoxorubicin) is a new analogue of the anthracycline, doxorubicin. This compound lacks the hydroxyl group at 4′ position on the amino sugar of the anthracycline. Phase II study was designed to determine the clinical response rate and to define the qualitative and quantitative toxicities of esorubicin in patients with adenocarcinoma of the pancreas. Fifty-eight patients with inoperable adenocarcinoma of the pancreas were entered on the study, 47 were evaluable for response, and 57 were evaluable for toxicity. The dose of esorubicin was 30 mg/m2 for good risk patients and 25 mg/m2 for poor risk patients every 21 days and administered IV push through a side arm of a running IV. Diphenhydramine, 50 mg is administered IM prior to the administration of the drug to block local venous reaction. Subsequent doses of esorubicin were modified according to granulocyte and platelet nadirs and the drug was not administered until recovery of platelets (> 100,000/ul) and wbc (> 3000/ul). Three partial responses, 20 stable, and 31 with increased disease were observed. Forty-seven had severe granulocytopenia (< 250), and two patients had severe thrombocytopenia (< 25,000). One patient experienced a decrease in left ventricular ejection fraction with a total dose of 180 mg/m2. The dose of esorubicin in this study demonstrated that the drug has minimal activity in adenocarcinoma of the pancreas but the toxicity is tolerable. Search should continue for single agents with activity in this disease.
Similar content being viewed by others
References
Arcamone F, Bernardi L, Patelli B, Giardino PD, Marco A, Casazza AM, Soranzo C, Pratesi G: Synthesis and antitumor activity of new daunorubicin and adriamycin analogues: Experientia 34:1255–1257, 1978
Salmon SE, Young L, Soehnlen B, Liu R: Antitumor activity of esorubicin in human clonogenic assay with comparisons to doxorubicin. J Clin Oncol 2:282–286, 1984
Cagnasso M (ed) Summary of preclinical studies of IMI-58 (4′ Deoxydoxorubicin) up to April 1981 (Farmitalia Carlo Erba)
Garewal HS, Robertone A, Salmon SE, Jones SE, Alberts DS, Brooks R: Phase I trial of 4′ deoxydoxorubicin (NSC No. 267469, Esorubicin). J Clin Oncol 2:1034–1039, 1984
Rozencweig M, Crespeigne N, Kenis Y: Phase I trial with 4′ deoxydoxorubicin (Esorubicin). Invest New Drugs 1:309–313, 1983
MacDonald JS, Ganderson LL, Conn I: Cancer of the pancreas. In: DeVita VT, Hellman S, Rosenberg SA (eds), Cancer, Principals and Practice of Oncology. Philadelphia, J.B. Lippincott, 1982, p 583
Hochster H, Green MD, Speyer JL, Wernz JC, Blum RH, Muggia FM: Activity of epirubicin in pancreatic carcinoma. Cancer Treat Rep 70:299–300, 1986
Schein PS, Lavin PT, Moertel CG: Randomized phase II clinical trial of adriamycin in advanced measurable pancreatic carcinoma. A GITSG Report, Cancer 42:19–22, 1978
Blayney DW, Goldbert DA, Leong LA, Carr BI, Doroshow J: Phase II trial of esorubicin in advanced pancreatic adenocarcinoma. Cancer Treat Rep 70:683–684, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vaughn, C.B., Salmon, S.E. & Fleming, T.R. Phase II evaluation of esorubicin (4′ deoxydoxorubicin) in pancreatic adenocarcinoma: A Southwest Oncology Group study. Invest New Drugs 8, 81–85 (1990). https://doi.org/10.1007/BF00216929
Issue Date:
DOI: https://doi.org/10.1007/BF00216929