Abstract
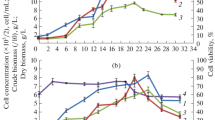
Two cell lines of Tabernaemontana divaricata cell suspension culture with different growth and alkaloid production profiles were transferred to the same medium. During 30 subcultures the changes in growth and alkaloid production were followed and compared to those of the original cell lines. The presence of NAA and BAP in the medium resulted in an increase of biomass and alkaloid yield. The effect on the growth proved to be stable during these 30 subcultures. Alkaloid production showed a maximum in the 4th subculture after the change of the medium, and stabilized on a higher level than found in the original cell lines. During some growth cycles also the activities of tryptophan decarboxylase (TDC), strictosidine synthase (SSS), and phenylalanineammonia-lyase (PAL) were measured. In both the original cell lines and the derived cell lines, growth and alkaloid production proved to be stable all through the experiment, although the derived cell lines had a period of adaptation to the new medium with increased productivity.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAA:
-
naphthaleneacetic acid
- BAP:
-
benzylaminopurine
- DW:
-
dry weight
- TDC:
-
tryptophan decarboxylase
- SSS:
-
strictosidine synthase
- PAL:
-
phenylalanineammonia-lyase
- PAT:
-
phenylalanineammonia-transaminase
References
Tabata M & Hiraoka N (1976) Variation of alkaloid production in Nicotiana rustica callus cultures. Physiol. Plant. 39: 19–23
Deus-Neumann B & Zenk MH (1984) Instability of indole alkaloid production in Catharanthus roseus cell suspension cultures. Planta Med. 50: 427–431
Morris P (1986) Long term stability of alkaloid productivity in cell suspension cultures of Catharanthus roseus. In: Morris P. Scragg AH, Stafford A & Fowler MW (Eds) Secondary Metabolism in Plant Cell Cultures (pp 257–262). Cambridge University Press, Cambridge
Morris P, Rudge K, Cresswell R & Fowler MW (1989) Regulation of product synthesis in cell cultures of Catharanthus roseus. V. Long-term maintenance of cells on production medium. Plant Cell Tiss. Org. Cult. 17: 79–90.
Shiio I & Ohta S (1973) Nicotine production by tobacco callus tissues and effect of plant growth regulators. Agr. Biol. Chem. 37: 1857–1864
Van der Heijden R (1988) Indole alkaloids in cell and tissue cultures of Tabernaemontana species. Ph. D. Thesis, University of Leiden, The Netherlands
Lindsey K & Yeoman MM (1983) The relationship beween growth rate, differentiation and alkaloid accumulation in cell cultures. J. Exp. Bot. 34: 1055–1065
Sierra MI, van der Heijden R, Schripsema J & Verpoorte R (1990) Alkaloid production in relation to differentiation in cell and tissue culture of Tabernaemontana pandacaqui. Submitted to Planta Med
Krikorian AD, Kelly K & Smith DL (1988) Hormones in tissue culture and micropropagation. In: Davies PJ (Ed) Plant Hormones and their Role in Plant Growth and Development (pp 593–613). Kluwer Acad. Publishers, Dordrecht
Davies PJ (1988) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (Ed) Plant Hormones and their Role in Plant Growth and Development (pp 1–23). Kluwer Acad. Publishers, Dordrecht
Morris P (1986) Regulation of product synthesis in cell cultures of Catharanthus roseus. II Comparison of production media. Planta Med. 52: 121–126
Furuya T, Kojima H & Syono K (1971) Regulation of nicotine biosynthesis by auxins in tobacco callus tissues. Phytochemistry 10: 1529–1532
Phillips R & Henshaw GG (1977) The regulation of synthesis of phenolics in stationary phase cell cultures of Acer pseudoplatanus. J. Exp. Bot. 28: 785–795
Zenk MH, El-Shagi H, Arens H, Stöckigt J, Weiler EW & Deus B (1977) Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharanthus roseus. In: Barz W, Reinhard E & Zenk MH (Eds) Plant Tissue Culture and its Biotechnological Application (pp 27–43). Springer-Verlag, Berlin, Heidelberg, New York
Stafford A, Smith L & Fowler MW (1985) Regulation of product synthesis in cell cultures of Catharanthus roseus (L) G. Don. Growth-related indole alkaloid accumulation in batch cultures. Plant Cell Tiss. Org. Cult. 4: 83–94
Roberts LW (1988) Evidence from would responses and tissue cultures. In: Roberts LW, Gahan PB & Aloni R (Eds) Vascular Differentiation and Plant Growth Regulators (pp 61–88). Springer-Verlag, Berlin Heidelberg
Masuda H, Fukuda H & Komamine A (1983) Changes in peroxidase isoenzyme patterns during tracheary element differentiation in a culture of single cells isolated from the mesophyll of Zinnia elegans. Z. Pflanzenphysiol 112: 417–426
Murashige T & Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Van der Heijden R, Lamping PJ, Out PP, Wijnsma R & Verpoorte R (1987) High-performance liquid chromatographic determination of indole alkaloids in a suspension cultures of Tabernaemontana divaricata. J Chromatogr. 396: 287–295
Da Cunha A (1987) The estimation of L-phenylalanine ammonia-lyase shows phenylpropanoid biosynthesis to be regulated by L-phenylalanine supply and availability. Phytochemistry 26: 2723–2727
Pennings EJM, Hegger I, van der Heijden R, Duine JA & Verpoorte R (1987) Assay of tryptophan decarboxylase from Catharanthus roseus plant cell cultures by highperformance liquid chromatography. Anal. Biochem. 165: 133–136
Pennings EJM, van der Bosch RA, van der Heijden R, Stevens L, Duine JA & Verpoorte R (1989) Assay of strictosidine synthase from plant cell cultures by highperformance liquid chromatography. Anal. Biochem. 176: 412–415
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Yamada Y & Mino M (1986) Instability of chromosomes and alkaloid content in cell lines derived from single protoplasts of cultured Coptis japonica cells. In: Current Topics in Developmental Biology, Vol 20 (pp 409–417). Yamada Science Foundation and Acad. Press Japan, Inc
Yamamoto H, Suzuki M, Suga Y, Fukui H & Tabata M (1987) Participation of an active transport system in berberine-secreting cultured cells of Thalictrum minus. Plant Cell Rep. 6: 356–359
Nakagawa K, Konagi A, Fukui H & Tabata M (1984) Release and crystallization of berberine in the liquid medium of Thalictrum minus cell suspension cultures. Plant Cell Rep. 3: 254–257
Deus-Neumann B & Zenk MH (1986) Accumulation of alkaloids in plant vacuoles does not involve an ion trap mechanism. Planta 167: 44–53
Tabata M, Yamamoto H, Hirakoa H, Marumoto Y & Konoshima M (1971) Regulation of nicotine production in tobacco tissue culture by plant growth regulators. Phytochemistry 10: 723–729
Tabata M, Mizukami H, Hiraoka N & Konoshima M (1974) Pigment formation in callus cultures of Lithospermun erythrorhizon. Phytochemistry 13: 927–932
Zenk MH, El-Shagi H & Schulte U (1975) Anthraquinone production by cell suspension cultures of Morinda citrifolia. Planta Med. Suppl. 79–101
Nakagawa K, Fukui H & Tabata M (1986) Hormonal regulation of berberine production in cell suspension cultures of Thalictrum minus. Plant Cell Rep. 5: 69–71
Jensen RA (1986) Tyrosine and phenylalanine biosynthesis: relationship between alternative pathways, regulation and subcellular location. In: Conn EE (Ed) Recent Advances in Phytochemistry. The Shikimic Acid Pathway (pp 57–81). Plenum Press, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sierra, M.I., van der Heijden, R., van der Leer, T. et al. Stability of alkaloid production in cell suspension cultures of Tabernaemontana divaricata during long-term subculture. Plant Cell Tiss Organ Cult 28, 59–68 (1992). https://doi.org/10.1007/BF00039916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00039916