Abstract
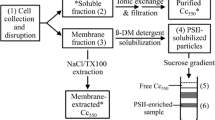
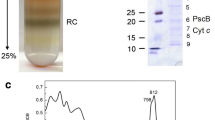
The membrane-bound photooxidizable cytochrome c-554 from Chloroflexus aurantiacus has been purified. The purified protein runs as a single heme staining band on SDS-PAGE with an apparent molecular mass of 43 000 daltons. An extinction coefficient of 28 ± 1 mM−1 cm−1 per heme at 554 nm was found for the dithionite-reduced protein. The potentiometric titration of the hemes takes place over an extended range, showing clearly that the protein does not contain a single heme in a well-defined site. The titration can be fit to a Nernst curve with midpoint potentials at 0, +120, +220 and +300 mV vs the standard hydrogen electrode. Pyridine hemochrome analysis combined with a Lowry protein assay and the SDS-PAGE molecular weight indicates that there are a minimum of three, and probably four hemes per peptide. Amino acid analysis shows 5 histidine residues and 29% hydrophobic residues in the protein. This cytochrome appears to be functionally similar to the bound cytochrome from Rhodopseudomonas viridis. Both cytochrome c-554 from C. aurantiacus and the four-heme cytochrome c-558-553 from R. viridis appear to act as direct electron donors to the special bacteriochlorophyll pair of the photosynthetic reaction center. They have a similar content of hydrophobic amino acids, but differ in isoelectric point, thermodynamic characteristics, spectral properties, and in their ability to be photooxidized at low temperature.
Similar content being viewed by others
Abbreviations
- LDAO:
-
lauryl dimethyl amine-N-oxide
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- mV:
-
millivolt
- Em.8 :
-
midpoint potential at pH 8.0
- ODV:
-
optical density x volume in ml
References
Alegria G and Dutton PL (1987) Construction and characterization of monolayer films of the reaction center cytochrome-c protein from Rhodopseudomonas viridis. In: Papa S, Chance B, Ernster L (eds) International Symposium on Cytochrome Systems: Molecular Biology and Bioenergetics, pp 601–608. New York: Plenum Press
Amesz J (1987) Primary electron transport and related processes in green photosynthetic bacteria. Photosynthetica 21: 225–235
Bartsch RG (1978) Cytochromes. In: Clayton RK and Sistrom WR (eds) The Photosynthetic Bacteria, pp 249–279. New York: Plenum Press
Berry EA and Trumpower BL (1987) Simultaneous determination of hemes a, b and c from pyridine hemochrome spectra. Anal Biochem 161: 1–15
Blankenship RE, Fieck R, Bruce BD, Kimaier C, Holten D and Fuller RC (1983) Primary photochemistry in the facultative green photosynthetic bacterium Chloroflexus aurantiacus. J Cellular Biochem 22: 251–261
Blankenship RE (1985) Electron transport in green photosynthetic bacteria. Photosynthe Res 6: 317–333
Blankenship RE, Huynh P, Gabrielson H and Mancino LJ (1985) Purification, physical properties and kinetic behavior of cytochrome c-554 from Chloroflexus aurantiacus. Biophys J 47: 2a
Blankenship RE, Brune DC, Freeman JM, King GH, McManus JD, Nozawa T, Trost J T and Wittmershaus BP (1988a) Energy trapping and electrotransfer in Chloroflexus aurantiacus. In: Olson JM, Ormerod JG, Amesz J, Stackebrandt E and Trüper HG (eds) Green Photosynthetic Bacteria, pp. 57–68. New York: Plenum Press
Blankenship RE, Trost JT and Mancino LJ (1988b) Properties of reaction centers from the green photosynthetic bacterium Chloroflexus aurantiacus. In: Breton J and Vermeglio A (eds) Structure of Bacterial Reaction Centers, pp 119–127. New York: Plenum Press
Bruce BD, Fuller RC and Blankenship RE (1982) Primary photochemistry in the facultatively aerobic green photosynthetic bacterium Chloroflexus aurantiacus. Proc Natl Acad Sci USA 79: 6532–6536
Case GD and Parson WW (1971) Thermodynamics of the primary and secondary photochemical reactions in Chromatium. Biochim Biophys Acta 253: 187–202
Creighton TE (1984) Proteins: Structure and Molecular Properties. p. 142. New York: W.H. Freeman and Co.
Deisemhofer J, Epp O, Miki K, Huber R and Michel H (1985) Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3 Å resolution. Nature 318: 618–624
Dracheva SM, Drachev LA, Zaberezhnaya SM, Konstantinov AA, Semenov AY and Skulachev VP (1986) Spectral, redox and kinetic characteristics of high-potential cytochrome c hemes in Rhodopseudomonas viridis Reaction Center. FEBS Lett 205: 41–46
Dracheva SM, Drachev LA, Konstantinov AA, Semenov AY, Skulachev VP, Arutjunjan AM, Shuvalov VA and Zaberezhnaya SM (1988) Electrogenic steps in the redox reactions catalyzed by photosynthetic reaction-centre complex from Rhodopseudomonas viridis. Eur J Biochem 171: 253–264
Drucker H, Trousil EB, Campbell LL, Barlow GH and Margoliash E (1970) Amino acid composition heme content and molecular weight of cytochrome c 3of Desulfovibrio desulficans and Desulfovibrio vulgaris. Biochem 9: 1515–1518
Dutton PL (1978) Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Meth Enzymol 54: 411–434
Feick RG and Fuller RC (1984) Topography of the photosynthetic apparatus of Chloroflexus aurantiacus. Biochemistry 23: 3693–3700
Foster JM, Redlinger TE, Blankenship RE and Fuller RC (1986) Oxygen regulation of development of the photosynthetic membrane system in Chloroflexus aurantiacus. J Bacteriol 167: 655–677
Freeman JC and Blankenship RE (1988) Purification and physical properties of membrane bound cytochrome c-554 from Chloroflexus aurantiacus. Photochem Photobiol 47: 41S
Fukushima A, Matsuura K, Shimada K and Satoh T (1988) Reaction center-B870 pigment protein complexes with bound cytochrome c-555 and c-551 from Rhodocyclus gelatinosus. Biochim Biophys Acta 933: 399–405
Goodhew CF, Brown KR and Pettigrew GW (1986) Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta 852: 288–294
Halsey YG and Byers B (1975) A large photoreactive particle from Chromatium vinosum chromatophores. Biochim Biophys Acta 387: 349–367
Holten D, Windsor MW, Parson WW and Thornber JP (1978) Primary photochemical processes in isolated reaction centers of Rhodopseudomonas viridis. Biochim Biophys Acta 501: 112–126
Kirmaier C and Holten D (1987) Primary photochemistry of reaction centers from the photosynthetic purple bacteria. Photosynthe Res 13: 225–260
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lin L and Thornber JP (1975) Isolation and partial characterization of the photochemical reaction center of Chromatium vinosum (strain D). Photochem Photobiol 22: 37–40
Loach PA (1970) Oxidation-reduction potentials, absorbance bands and molar absorbance of compounds used in biochemical studies. In: Sober HA (ed) Handbook of Biochemistry: Selected Data for Molecular Biology, pp C-281-C-287. Cleveland: The Chemical Rubber Co.
McManus JD, Trost JT and Blankenship RE (1988) Kinetic behavior and N-terminal amino acid sequence of Auracyanin. Biophys J 53: 268a
Meyer TE, Bartsch RG and Kamen MD (1971) Cytochrome c3: A class of electron transfer heme proteins found in both photosynthetic and sulfate-reducing bacteria. Biochim Biophys Acta 245: 453–464
Meyer TE and Kamen MD (1982) New perspectives on c-type cytochromes. Adv Protein Chem 35: 105–212
Nozawa T, Trost JT, Fukada T, Hatano M, McManus JD and Blankenship RE (1987) Properties of the reaction center of the thermophilic purple photosynthetic bacterium Chromatium tepidum. Biochim Biophys Acta 894: 468–476
Olson JM (1980) Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta 594: 33–51
Ovchinnikov YA, Abdulaev NG, Zolotarev AS, Shmukler BE, Zargarov AA, Kutuzov MA, Telezhinskaya IN and Levina NB (1988a) Photosynthetic reaction centre of Chloroflexus aurantiacus. I. Primary structure of the L-subunit. FEBS Lett 231: 237–242
Ovchinnikov YA, Abdulaev NG, Shmukler BE, Zargarov AA, Kutuzov MA, Telezhinskaya IN, Levina NB and Zolotarev AS (1988b) Photosynthetic reaction centre of Chloroflexus aurantiacus. Primary structure of the M-subunit. FEBS Lett 232: 364–368
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83: 346–356
Pettigrew GW and Moore GR (1987) Cytochromes c Biological Aspects. Berlin: Springer-Verlag
Pierson BK (1985) Cytochromes in Chloroflexus aurantiacus grown with and without oxygen. Arch Microbiol 143: 260–265
Pierson BK and Thornber JP (1983) Isolation and spectral characterization of photochemical reaction centers from the thermophilic green bacterium Chloroflexus aurantiacus strain J-10-fl. Proc Natl Acad Sci USA 80: 80–94
Pierson BK and Castenholz RW (1974) A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol 100: 5–24
Postgate JR (1956) Cytochrome c 3and Desulphoviridin: pigments of the anaerobe Desulphovibrio desulphuricans. J Gen Microbiol 14: 545–572
Prince RC, Leigh JS and Dutton PL (1976) Thermodynamic properties of the reaction center of Rhodopseudomonas viridis, in vivo measurements of the reaction center bacteriochlorophyll primary acceptor intermediary electron carrier. Biochim Biophys Acta 440: 622–636
Prince RC, Linkletter SJG and Dutton PL (1981) The thermodynamic properties of some commonly used oxidation-reduction mediators, inhibitors and dyes as determined by polarography. Biochim Biophys Acta 635: 132–148
Reeck G (1970) Amino acid compositions of selected proteins. In: Sober HA (ed) Handbook of Biochemistry Selected Data for Molecular Biology, pp C281-C-287. Cleveland: The Chemical Rubber Co.
Shill DA and Wood PM (1984) A role for cytochrome c 2in Rhodopseudomonas viridis. Biochim Biophys Acta 764: 1–7
Shiozawa JA, Lottspeich F and Feick R (1987) The photochemical reaction center of Chloroflexus aurantiacus is composed of two structurally similar polypeptides. Eur J Biochem 167: 595–600
Thomas PE, Ryan D and Levin W (1976) An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem 75: 168–176
Tiede DM, Prince RC and Dutton PL (1976) EPR and optical spectroscopic properties of the electron carrier intermediate between the reaction center bacteriochlorophylls and the primary acceptor in Chromatium vinosum. Biochim Biophys Acta 449: 447–467
Trosper TL, Benson DL, and Thornber JP (1977) Isolation and spectral characteristics of the photochemical reaction center of Rhodopseudomonas viridis. Biochim Biophys Acta 460: 318–330
Trost JT, McManus JD, Freeman JC, Ramakrishna BL and Blankenship RE (1988) Auracyanin: A blue copper protein from the green photosynthetic bacterium Chloroflexus aurantiacus. Biochemistry 27: 7858–7863
Wakabayashi S, Matsubara H, Kim CH and King TE (1982) Structural studies of bovine heart cytochrome c 1. J Biol Chem 257: 9335–9344
Weyer KA, Schäfer W, Lottspeich F and Michel H (1987a) The cytochrome subunit of the photosynthetic reaction center from Rhodopseudomonas viridis is a lipoprotein. Biochemistry 26: 2909–2914
Weyer KA, Lottspeich F, Gruenberg H, Land F, Osterhelt D and Michel H (1987b) Amino acid sequence of the cytochrome subunit of the photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J 6: 2197–2202
Widger WR, Cramer WA, Herrmann RA and Trebst A (1984) Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b 6f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci USA 81: 674–678
Willey DL, Auffret AD and Gray JC (1984) Structure and topology of cytochrome f in pea chloroplast membranes. Cell 36: 555–562
Woese CR (1987) Bacterial evolution. Microbiol Rev 51: 221–271
Wynn RM, Redlinger TE, Foster JM, Blankenship RE, Fuller RC, Shaw RW and Knaff DB (1987) Electron transport chins of phototrophically and chemotrophically grown Chloroflexus aurantiacus. Biochim Biophys Acta 891: 216–226
Zannoni D (1987) The interplay between photosynthesis and respiration in facultative anoxygenic phototrophic bacteria. In: Papa S, Chance B and Ernster L (eds) International Symposium on Cytochrome Systems: Molecular Biology and Bioenergetics pp 575–583. New York: Plenum Press
Zannoni D and Ingledew WJ (1985) A thermodynamic analysis of the plasma membrane electron transport components in photoheterotrophically grown cells of Chloroflexus aurantiacus: an optical and electron paramagnetic resonance study. FEBS Lett 193: 93–98
Zannoni D and Venturoli G (1988) The mechanism of photosynthetic electron transport and energy transduction by membrane fragments from Chloroflexus aurantiacus. In: Olson JM, Ormerod JG, Amesz J, Stackebrandt E and Trüper HG (eds) Green Photosynthetic Bacteria, pp 135–143, New York: Plenum Press
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Freeman, J.C., Blankenship, R.E. Isolation and characterization of the membrane-bound cytochrome c-554 from the thermophilic green photosynthetic bacterium Chloroflexus aurantiacus . Photosynth Res 23, 29–38 (1990). https://doi.org/10.1007/BF00030060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00030060