Abstract
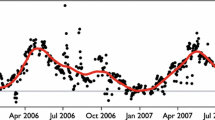
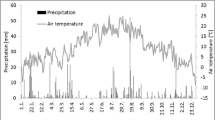
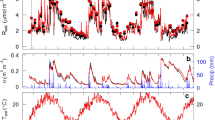
Soil surface CO2 flux was measured in hollow and hummock microhabitats in a peatland in north central Minnesota from June to October in 1991. We used a closed infrared gas exchange system to measure soil CO2 flux. The rates of CO2 evolution from hummocks (9.8 ± 3.5 g m−2 d−1, [mean ± SE]) were consistently higher than those from hollows (5.4 ± 2.9 g m−2 d−1) (the hummock values included the contribution of moss dark respiration, which may account for 10–20% of the total measured flux). The soil CO2 flux was strongly temperature-dependent (Q10 ≈ 3.7) and appeared to be linearly related to changes in water table depth. An empirical multiplicative model, using peat temperature and water table depth as independent variables, explained about 81% of the variance in the CO2 flux data. Using the empirical model with measurements of peat temperature and estimates of hollow/hummock microtopographic distribution (relative to water table elevation), daily rates of “site-averaged” CO2 evolution were calculated. For the six-month period (May–October), the total soil CO2 released from this ecosystem was estimated to be about 1340 g CO2 m−2.
Similar content being viewed by others
References
Anderson JM (1973) Carbon dioxide evolution from two temperate, deciduous woodland soils. J. Appl. Ecology 10: 361–378
Baldocchi DD, Verma SB, Matt DR & Anderson DE (1986) Eddy-correlation measurements of carbon dioxide efflux from the floor of a deciduous forest. J. Appl. Ecology 23: 967–975
Billings WD (1987) Carbon balance of Alaskan tundra and Taiga ecosystems: past, present and future. Quat. Sci. Rev. 6: 165–177
Billings WD, Peterson KM, Shaver GR & Trent AW (1977) Root growth, respiration, and carbon dioxide evolution in an arctic tundra soil. Arct. Alp. Res. 9: 129–8137
Billings WD, Luken JO, Mortensen DA & Peterson KM (1982) Arctic tundra: a source or sink for atmospheric carbon dioxide in a changing environment? Oecologia (berl) 53: 7–11
Billings WD, Luken JO, Mortensen DA & Peterson KM (1983) Increasing atmospheric carbon dioxide: possible effects on arctic tundra. Oecologia (berl) 58: 286–289
Bonan GB (1991) Atmosphere-biosphere exchange of carbon dioxide in boreal forests. J. Geophys. Res. 96: 7301–7312
Bunnell FL & Tait DEN (1974) Mathematical simulation models of decomposition processes. In: Holding AJ, Heal OW, MacLean Jr. SF & Flanagan PW (Eds) Soil Organisms and Decomposition in Tundra (pp 207–255). Tundra Biome Steering Committee, Stockholm
Bunnell FL, Tait DEN, Flanagan PW & Van Cleve K (1977) Microbial respiration and substrate weight loss — I. A general model of the influences of abiotic variables. Soil Biol. Biochem. 9: 33–40
Carlyle JC & Than UB (1988) Abiotic controls of soil respiration beneath an eighteenyear-oldPinus radiata stand in south-eastern Austr. J. Ecol. 76: 654–662
Edwards NT (1975) Effects of temperature and moisture on carbon dioxide evolution in a mixed deciduous forest floor. Soil Sci. Soc. Am. Proc. 39: 361–365
Fan S-M, Wofsy SC, Bakwin PS & Jacob DJ (1990) Atmosphere-biosphere exchange of CO2 and O3 in the central Amazon Forest. J. Geophys. Res. 95: 16851–16864
Flanagan PW & Veum AK (1974) Relationships between respiration, weight loss, temperature and moisture in organic residues on tundra. In: Holding AJ, Heal OW MacLean Jr. SF & Flanagan PW (Eds) Soil Organisms and Decomposition in Tundra (pp249–277). Tundra Bioms Steering Committee, Stockholm
Flanagan PW & Van Cleve K (1977) Microbial biomass, respiration and nutrient cycling in a black spruce taiga ecosystem, soil organisms as components of ecosystems. Ecol. Bull. 25: 261–273
Gorham E (1991) Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1: 182–195
Grahammer K (1989) Respiration of soil and vegetation in grassland. M. S. Thesis, Dept. of Agronomy, Univ. of Nebraska, Lincoln
Hartman RK & Gay LW (1981) Improvements in the design and the calibration of temperature measurement systems. Processings of the 15th Conference on Agricultural and Forest Meteorology, 210 p
Heal OW (1979) Decomposition and nutrient release in even-aged plantations. In: Ford ED, Malcolm DC & Atterson J (Eds) The Ecology of Even-aged Forest Plantations (pp 257–291). Proceedings of the Meeting of Division I., International Union of Forest Research Organizations, Edinburgh 1978. Institute of Terrestrial Ecology, Cambridge
Keller M, Kaplan WA & Wofsy SC (1986) Emission of N2O, CH4 and CO2 from tropical forest soils. J. Geophys. Res. 91: 11791–11802
Kucera CL & Kirkham DR (1971) Soil respiration studies in tallgrass prairie in Missouri. Ecology 52: 912–914
Luken JO & Billings WD (1985) The influence of microtopographic heterogeneity on carbon dioxide efflux from a subarctic bog. Holarc. Ecol. 8: 306–312
Medina E & Zelwer M (1972) Soil respiration in tropical plant communities. In: Golley PM & Golley FB (Eds) Papers from a Symposium on Tropical Ecology with an Emphasis on Organic Productivity (pp 245–269) Univ. Georgia, Athens
Moore TR (1986) Carbon dioxide evolution from subarctic peatlands in eastern Canada. Arct. Alp. Res. 18: 189–193
Moore TR & Knowles R (1987) Methane and carbon dioxide evolution from subarctic fens. Can. J. Soil Sci. 67: 77–81
Moore TR and Knowles R (1989) The influence of water table levels on methane and carbon dioxide emissions from peatland soils. Can. J. Soil Sci. 69: 33–38
Nakayama FS (1990) Soil respiration. Remote Sens. Rev. 5: 311–321
Norman JM, Garcia R & Verma SB (1990) Soil surface CO2 fluxes on the Konza Prairie. Proceedings of American Meteorological Society, pp 29–31
Norman JM, Garcia R & Verma SB (1992) Soil surface CO2 fluxes and the carbon budget. J. Geophys. Res. (in press)
Oberbauer SF, Oechel WC & Riechers GH (1986) Soil respiration of Alaskan tundra at elevated atmospheric carbon dioxide concentrations. Plant Soil 96: 145–48
Oberbauer SF, Gillespie CT, Cheng W, Gebauer R, Sala Serra A & Tenhunen JD (1992) Environmental effect on CO2 efflux from riparian tundra in the northern foothills of the Brooks Range, Alaska, USA (submitted)
Oechel WC (1976) Seasonal patterns of temperature response of CO2 flux and acclimation in arctic mosses growingin situ. Photosynthetica 10: 447–456
Oechel WC (1989) Nutrient and water flux in a small Arctic watershed: an overview. Holarc. Ecol. 12: 229–237
Peterson KM, Billings WD & Reynolds DN (1984) Influence of water table and atmospheric CO2 concentration on the carbom balance of arctic tundra. Arc. Alp. Res. 16: 331–335
Peterson KM & Billings WD (1975) Carbon dioxide flux from tundra soils and vegetation as related to temperature at Barrow, Alaska. The American Midland Naturalist 94: 88–98, 177
Poole DK & Miller PC (1982) Carbon dioxide flux from three arctic tundra types in northcentral Alaska, USA. Arct. Alp. Res. 14: 27–32
Reiners WA (1968) Carbon dioxide evolution from the floor of three Minnesota forests. Ecology 49: 471–483
Schlentner RE & Van Cleve K (1985) Relationships between CO2 evolution from soil, substrate temperature, and substrate moisture in four mature forest types in interior Alaska. Can. J. For. Res. 15: 97–106
Schlesinger WH (1977) Carbon balance in terrestrial detritus. Annu. Rev. Ecol. Syst. 8: 51–81
Schulze E (1967) Soil respiration of tropical vegetation types. Ecology 48: 652–653
Singh JS & Gupta SR (1977) Plant decomposition and soil respiration in terrestrial ecosystems. The Botanical Review 43: 449–528
Svensson BH (1980) Carbon dioxide and methane fluxes from the ombrotrophic parts of a subarctic mire. In: Sonesson M (Ed) Ecology of a Subarctic Mire. Ecol. Bull. 30: 235–250
Verry ES (1975) Streamflow chemistry and nutrient yields from upland-peatland watersheds in Minnesota. Ecology 56: 1149–1157
Verry ES (1988) Hydrology of wetlands and men's influence on it. In: Symposium on the Hydrology of Wetlands in Temperate and Cold Regions, Vol. 2, 6–8 June, 1988, Joensuu, Finland. Publications of the Academy of Finland (Helsinki) 5:41–61
Wanner H (1970) Soil respiration, litter fall and productivity of tropical rain forest. J. Ecol. 58: 543–547
Author information
Authors and Affiliations
Additional information
Published as Paper No. 9950, Journal Series, Nebraska Agricultural Research Division, University of Nebraska, Lincoln, NE, USA.
Rights and permissions
About this article
Cite this article
Kim, J., Verma, S.B. Soil surface CO2 flux in a Minnesota peatland. Biogeochemistry 18, 37–51 (1992). https://doi.org/10.1007/BF00000425
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00000425