Abstract
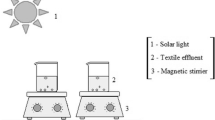
Different parameters were investigated to evaluate their effect on the process removal efficiency of reactive dye from simulated spent reactive dye bath, by solar / TiO2 / H2O2, including H2O2 concentration, TiO2 loading and pH. As a result 99% of reactive dye can be removed at a TiO2 loading of 400mg/l, H2O2 concentration of 150 mg/l and of pH: 5.2. The effect of photo-catalytic deactivation of TiO2 on reactive dye removal was studied for ten number of cycles, and found that the extent of deactivation was high for each consecutive repeated use.
Similar content being viewed by others
References
Buxton G. V., Greenstock C., Helman W. P. and Ross A. B., (1988). Critical review of rate constants for reaction of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J. Phys. Chem. Ref. Dat., 17, 513–886.
Anonymous, (1998). Hand book on photo chemical oxidation processes. EPA / 625 /B-98 / 004, EPA.
Kanmani S. and Thana sekarn K., (2003). Decolourisation of industrial wastewaters of textile dyeing industry by photo catalysis, Indian J. Chem. Technol., 10, 53–59.
Laszlo J. A., (1994). Removing acid dyes from textile wastewater using biomass for decolorization. Am. Dyest. Rep., 83, 17–21.
Leonas K. K. and Leonas M. L., (1994). Textile process waste water permits: an update and strategies. Am. Dyest. R., 83, 26–34.
Swadesh K. Chaudhuri and Sur B., (2000). Oxidative decolorization of reactive dye solution using Flyash as catalyst. J. Environ. Eng., 126, 583–594.
Turchi C. S., Klausner J. F., Goswami D. Y. and Marchand E., (1994). Field test results for the solar photo catalytic detoxification of fuel contaminated ground water. Chemical oxidation technologies for the nintties, W. W. Ecken Felder, A. R. Bowers and J. A. Roth (Eds). Technomic publishing co., Inc. Lancaster, Pennsylvanla, 3.
Vogel A. I., (1978). A text book of quantitative inorganic analysis. 4th. Ed., Longman Scientific and Technical, Harlow, U.K., 381–382.
Yeh R. Y. L., Liu R. L. H., Chiu H. M. and Hung Y. T., Compartive study of adsorption capacity of various adsorbents for treating dye wastewaters, Int. J. Environ. Stud. 44, 259–254.
Zollinger H., (1987). Color chemistry-synthesis, properties and applications of organic dyes and pigments. VCH Publishers, Newyork, 92–102.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, S.S., Kotaiah, B. Decolorization of simulated spent reactive dye bath using solar / TiO2 / H2O2 . Int. J. Environ. Sci. Technol. 2, 245–251 (2005). https://doi.org/10.1007/BF03325883
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03325883