Summary
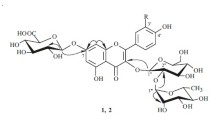
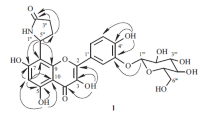
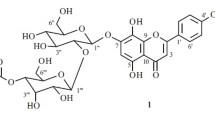
A new flavonol glycoside has been isolated from the flower petals ofHibiscus sabdariffa, and has been named Hibiscitrin. Its aglycone, Hibiscetin is a hexahydroxy flavonol forming a heptaacetyl derivative on acetylation. When decomposed with boiling 50% alkali, its heptamethyl ether produces trimethyl gallic acid, indicating thereby the existence of hydroxyl groups in 3′, 4′ and 5′ positions in hibiscetin. On oxidation withp-benzoquinone, the pigment yields the corresponding quinone, and hence it should contain two hydroxyl groups in positions 5 and 8. It resembles gossypetin in many of its properties especially the colour changes with alkaline buffer solutions, and also occurs along with it in the flowers. It is, therefore, expected that the benzopyrone part of hibiscetin would be just the same as in gossypetin, and hence it is assigned the structure of 3∶ 5∶ 7∶ 8∶ 3′∶ 4′∶ 5′-heptahydroxy-flavone.
Similar content being viewed by others
References
PerkinJ. C. S., 1909,95, 1855.
Neelakantam, Rao and SeshadriProc. Ind. Acad. Sci., 1941,14, 105.
— and SeshadriIbid.,, 1936,4, 54.
Rao and SeshadriIbid.,, 1939,9, 177.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rao, P.S., Seshadri, T.R. Isolation of hibiscitrin from the flowers ofHibiscus sabdariffa: Constitution of Hibiscetin. Proc. Indian Acad. Sci. (Math. Sci.) 15, 148–153 (1942). https://doi.org/10.1007/BF03051846
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03051846