Abstract
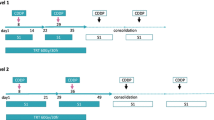
Non-small-cell lung cancer (NSCLC) has one of the highest death rates among the various forms of cancer. In attempts to improve on this unsatisfactory outcome, different radiation schedules and chemotherapy agents have been examined in phase II or III studies. These have led to modest improvements in local control and survival, but combined therapies are associated with substantial hematologic toxicity. In this phase II study, 80 consecutive stage IIIA or IIIB NSCLC patients were treated with concomitant chemotherapy and twice-a-day irradiation in a total dose of 60 Gy in 1.5 Gy fractions. Patients scheduled for surgery received 45 Gy only. Paclitaxel (30 mg/m2) on days 1–4 and cisplatin (100 mg/m2) on day 5 were administered in the first and fourth weeks of treatment. Granulocyte colony stimulating factor (30 ng/m2) was given on days 10–15. The local control, the 1- and 2-year survival rates and the occurrence of acute hematologic toxicity in the non-surgically treated patients were examined. Fifty-two patients were treated without and 28 with surgery. Among the non-surgically treated cases, 43 were evaluable for response and 47 for acute toxicity during a median follow-up of 22 months. The rate of local control was 65% (28/43), and the 1- and 2-year survival rates proved to be 68% and 48%, respectively, with a median survival of 28 months. Severe acute grade 3–4 toxicities included grade 4 leukopenia in 6 cases (13%), grade 3 leukopenia in 4 cases (9%), grade 3 esophagitis in 3 cases (6%) and grade 3 anemia in 3 cases (6%). Our results and the relevant data from the literature support the application of twice-a-day irradiation with concomitant chemotherapy in stage IIIA and IIIB NSCLC. Local control and survival were improved relative to once-a-day irradiation with sequential or concomitant chemotherapy.
Similar content being viewed by others
References
Arriagada R, Le Chevalier T, Quoix E, et al: ASTRO Plenary: Effect of chemotherapy on locally advanced non-small cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d’Étude et Traitment des Cancers Bronchiques), FNCLC (Federation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys 20:1183–1190, 1991
Byhardt RW, Pajak TF, Emami B, et al: A phase I/II study to evaluate accelerated fractionation via concomitant boost for squamous, adeno, and large cell carcinoma of the lung: report of Radiation Therapy Oncology Group 84-07. Int J Radiat Oncol Biol Phys 26:459–468, 1993
Byhardt RW, Scott CB, Ettinger DS, et al: Concurrent hyperfractionated irradiation and chemotherapy for unresectable nonsmall cell lung cancer. Results of Radiation Therapy Oncology Group 90-15. Cancer 75:2337–2344, 1995
Cancer Therapy Evaluation Program. Common Toxicity Criteria, Version 2.0. (1997) http://ctep.info.nih.gov
Choy H, Devore RF III, Hande KR, etal: A phase II study of paclitaxel, carboplatin, and hyperfractionated radiation therapy for locally advanced inoperable non-small-cell lung cancer (a Vanderbilt Cancer Center Affiliate Network Study). Int J Radiat Oncol Biol Phys 47:931–937, 2000
Clamon G, Herndon J, Cooper R, et al: Radiosensitization with carboplatin for patients with unresectable stage III nonsmall-cell lung cancer: a phase III trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol 17: 4–11, 1999
Cox JD, Azarnia N, Byhardt RW, et al: A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0 Gy to 79.2 Gy: possible survival benefit with greater than or equal to 69.6 Gy in favorable patients with Radiation Therapy Oncology Group stage III non-small-cell lung carcinoma: report of Radiation Therapy Oncology Group 83-11. J Clin Oncol 8:1543–55, 1990
Cox JD, Azarnia N, Byhardt RW, et al: Altered fractionation for non-small-cell carcinoma of the lung. Chest 96:68S-69S, 1999
Dillman RO, Seagren SL, Propert KJ, et al: A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 323: 940–945, 1990
Greenlee R, Hill-Harmon MB, Murray T, Thun M: Cancer statistics, 2001. CA Cancer J Clin 51:15–36, 2001
Hazuka MB, Crowley JJ, Bunn PA Jr, et al: Daily low—dose cisplatin plus concurrent high-dose thoracic irradiation in locally advanced unresectable non-small-cell lung cancer: results of a phase II Southwest Oncology Group Study. J Clin Oncol 12:1814–1820, 1994
International Commission on Radiation Units and Measurements. ICRU Report 50. Prescribing, recording, and reporting photon beam therapy. Bethesda, MD, 1993
International Commission on Radiation Units and Measurements (eds: Wambersie A, Landberg T) ICRU Report 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50) Bethesda, MD, 1999
Jeremic B, Shibamoto Y, Acimovic L, et al: Hyperfractionated radiation therapy and concurrent low-dose, daily carboplatin/etoposide with or without weekend carboplatin/etoposide chemotherapy in stage III non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys 50: 19–25, 2001
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Ass 54: 457–481, 1958
Kelly K, Hazuka M, Pan Z, et al: A phase I study of daily carboplatin and simultaneous accelerated, hyperfractionated chest irradiation in patients with regionally inoperable nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 40:559–567, 1998
Komaki R, Scott C, Ettinger D, et al: Randomized study of chemotherapy/radiation therapy combinations for favorable patients with locally advanced inoperable nonsmall cell lung cancer: Radiation Therapy Oncology Group (RTOG) 92-04. Int J Radiat Oncol Biol Phys 38:149–155, 1997
Langer CJ, Curran WJ, Keller SM, et al: Report of phase II trial of concurrent chemoradiotherapy with radical thoracic irradiation (60 Gy), infusional fluorouracil, bolus cisplatin and etoposid for clinical stage IIIB and bulky IIIA non-small cell lung cancer. Int J Radiat Oncol Biol Phys 26: 469–478, 1993
Martel MK, Ten Haken RK, Hazuka MB, et al: Estimation of tumor control probability model parameters from 3D dose distributions of non-small cell lung cancer patients. Lung Cancer 24: 31–37, 1999
Mehta MP, Tannehill SP, Adak S, et al: Phase II trial of hyperfractionated accelerated radiation therapy for nonresectable non-small-cell lung cancer: results of Eastern Cooperative Oncology Group 4593. J Clin Oncol 11: 3518–3523, 1998
Perez CA, Pajak TF, Rubin P, et al: Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 59: 1874–1881, 1987
Pisch J, Berson AM, Malamud S, et al: Chemoradiation in advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 33: 183–188, 1995
Saunders M, Dische S, Barrett A, et al: Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicenter trial. CHART Steering Committee. Lancet 350:161–165, 1997
Sause WT, Scott C, Taylor S, et al: Phase II trial of combination chemotherapy and irradiation in non-small-cell lung cancer, Radiation Therapy Oncology Group 88-04. Am J Clin Oncol 15:163–167, 1992
Sause W, Kolesar P, Taylor SIV, et al: Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 117:358–364, 2000
Schaake-Koning C, van den Bogaert W, Dalesio O, et al: Effects of concomitant cisplatin and radiotherapy on inoperable nonsmall-cell lung cancer. N Engl J Med 326: 524–530, 1992
Trott KR, Kummermehr J. The time factor and repopulation in tumors and normal tissues. Semin Radiat Oncol 3: 115–125, 1993
Withers HR, Taylor JM, Maciejewski B: The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27: 131–146, 1988
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pisch, J., Moskovitz, T., Ésik, O. et al. Concurrent paclitaxel-cisplatin and twice-a-day irradiation in stage IIIA and IIIB NSCLC shows improvement in local control and survival with acceptable hematologic toxicity. Pathol. Oncol. Res. 8, 163–169 (2002). https://doi.org/10.1007/BF03032389
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03032389