Abstract
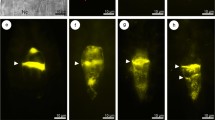
Anatomical differences between embryogenic and non-embryogenic calli ofPimpinella brachycarpa were investigated by light microscopy and electron microscopy. Initial callus tissue emerged from expiants after 14 d of culturing. The embryogenie calli (EC) were firm, rather opaque, and light yellow in color. The cells usually formed small, compact clusters. Nonembryogenic calli (NEC), however, were friable, semitransparent, and yellow or gray. These formed relatively larger and loosely held clusters. Scanning electron microscopy showed that EC were composed of individual compact and spherical cells that were rather regular in size and approximately 20 µm long. All were tightly held together and appeared to organize globular embryos. In contrast, the NEC comprised elongated and loosely held cells that were approximately 50 µm long. Tubular and u-shaped NEC cells protruded irregularly, and were of varying heights along the cell aggregates. Transmission electron microscopy of the EC revealed typical eukaryotic cytoplasmic components, including nuclei, mitochondria, and vacuoles in the cytoplasm enclosed by an electron-transparent cell wall. Based on the numerous ribosomes within the cytoplasm, these cells appeared to be well-organized and metabolically active. The NEC cells were much larger and more highly vacuolated than those of the EC. In ultrathin sections, the former seemed to be almost devoid of other cellular contents except for plastids and nuclei. Furthermore, EC and NEC showed different regeneration capacities in their somatic embryo formation. Most EC produced hyperhydric somatic embryos, followed by normal somatic embryos; whereas only a few shooted or rooted somatic embryos arose from the NEC.
Similar content being viewed by others
Literature cited
Albarran J, Bertrand B, Lartaud M, Etienne H (2005) Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffeeaarabica) somatic embryo. Plant Cell Tiss Org Cult81: 27–36
Charbit E, Legavre T, Lardet L, Bourgeois E, Ferriere N, Carron MP (2004) Identification of differentially expressed cDNA sequences and histological characteristics ofHevea brasiliensis calli in relation to their embryogenic and regenerative capacities. Plant Cell Rep22: 539–548
Debergh P, Aitken-Christie J, Cohen D, Grout B, von Arnold S, Zimmerman R, Ziv M (1992) Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tiss Org Cult30: 135–140
Lee EJ, Mobin M, Hahn EJ, Paek KY (2006) Effects of sucrose, inoculum density, auxins, and aeration volume on cell growth ofCymnema sylvestre. J Plant Biol49: 427–431
Ibaraki Y, Kaneko Y, Kurata K (1998) Evaluation of embryogenic potential of cell suspension culture by texture analysis. Trans Amer Soc Agric Engr41: 247–252
Ibaraki Y, Kurata K (1997) Image analysis base quantification of cells in suspension cultures for producing somatic embryos. Environ Contr Biol35: 63–67
Ibaraki Y, Kurata K (2001) Automation of somatic embryo production. Plant Cell Tiss Org Cult65: 179–199
Ikeda-lwai M, Satoh S, Kamada H (2002) Establishment of a reproducible tissue culture system for the induction ofArabidopsis somatic embryos. J Exp Bot53: 1575–1580
Jimenez VM, Bangerth F (2001) Endogenous hormone levels in initial expiants and in embryogenie and nonembryogenic callus cultures of competent and non-competent wheat genotypes. Plant Cell Tiss Org Cult67: 37–46
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol27: 137–138
Kaur N, Jain H, Mann P, Gupta AK, Singh RA (1992) Comparison of properties of invertases and inulinase from chicory. Plant Physiol Biochem304: 445–450
Kim JC, Chang MY, Son SI, Heo SJ (2001) Thidiazuron required for efficient somatic embryogenesis from suspension-cultured cells ofPimpinella brachycarpa. J Plant Biol44: 224–230
Moon HK, Yang Y, Lee SK (1994) Rapid micropropagation ofPimpinella brachycarpa via somatic embryogenesis. Kor J Plant Tiss Cult21: 85–90
Moon HY, Kim JA, Park SY, Kim YW, Kang HD (2006) Somatic embryogenesis and plantlet formation from a rare and endangered tree species,Oplopanax elatus. J Plant Biol49: 320–325
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant15: 473–497
Nabors MW, Heyser JW, Dykes TA, DeMott KJ (1983) Long-duration, high-frequency plant regeneration from cereal tissue cultures. Planta157: 385–391
Oinam GS, Kothari SL (1995) Totipotency of coleoptile tissue in Indica rice (Oryza sativa L. cv. CH 1039). Plant Cell Rep14: 245–248
Profumo P, Gastaldo P, Dameri RM, Caffaro L (1986) Histological study of calli and embryoids of leaf expiants ofAesculus hippocastanum L. J Plant Physiol126: 97–103
Profumo P, Gastaldo P, Rascio N (1987) Ultrastructural study of different types of callus from leaf expiants ofAesculus hippocastanum L. Protoplasma138: 89–97
Quiroz-Figueroa FR, Mendez-Zeel M, Sanchez-Teyer F, Rojas-Her-rera R, Loyola-Vargas VM (2002) Differential gene expression in embryogenic and nonembryogenic clusters from cell suspension cultures ofCoffea arabica L. J Plant Physiol159: 1267–1270
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol17: 208–212
Samaj J, Baluska F, Bobak M, Volkmann D (1999) Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep18: 369–374
Sharp WR, Sondahl MR, Caldas LS, Maraffa SB (1980) The physiology of in vitro asexual embryogenesis. Hort Rev2: 268–310
Smith SM, Street HE (1974) The decline of embryogenic potential as callus and suspension cultures of carrot(Daucus carota L.) are serially subcultured. Ann Bot38: 223–241
Son SI, Kim JC (1999) Cloning and characterization of home-odomain-Zip gene,Phc5, in embryogenic callus derived fromPimpinella brachycarpa suspension cultured cells. Kor J Plant Tiss Cult26: 121–126
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res26: 31–43
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: Improving somatic embryo quality. Plant Cell Tiss Org Cult74: 15–35
Stirn S, Hopstock A, Lorz H (1994) Bioreactor cultures of embryogenic suspensions of barley(Hordeum vulgare L.) and maize (Zeamays L.). J Plant Physiol144: 209–214
Stirn S, Jacobsen HJ (1987) Marker proteins for embryogenic differentiation patterns in pea callus. Plant Cell Rep6: 50–54
Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis,In TA Thorpe, ed, In Vitro Embryogenesis in Plants. Kluwer Academic, Dordrecht, pp 205–247
Zhang C, Chi CM, Staba EJ, Cooke TJ, Hu WS (1996) Application of image analysis to fed-batch cultures of somatic embryos. In Vitro Cell Dev Biol32: 190–198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Na, H., Kim, K.W., Kwack, Y. et al. Comparative anatomy of embryogenic and non-embryogenic calli frompimpinella brachycarpa . J. Plant Biol. 50, 344–350 (2007). https://doi.org/10.1007/BF03030665
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03030665