Abstract
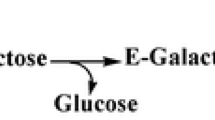
Oligosaccharides produced by submerged cultures ofC. africana andC. sorghi were isolated by semipreparative HPLC. Structure of 6-O-β-d-fructofuranosyl-d-glucopyranose (blastose), 1,6-bis-O-(β-d-fructofuranosyl)-α-d-glucopyranoside (neokestose) and two sugar alcohols, 1-O-β-d-fructofuranosyl-d-mannitol (fructosylmannitol) and 1,6-bis-O-(β-d-fructofuranosyl)-d-mannitol (bisfructosylmannitol) was determined by NMR spectrometry. MALDI TOF MS analysis revealed molecular ions [M+Na]+ that indicate the presence of other tetra- and pentasaccharides (m/z=689.4 and 851.5, respectively) and corresponding sugar alcohol (m/z=691.4). Rapid conversion of sucrose into series of oligosaccharides and corresponding sugar alcohols was observed in all tested strains.
Similar content being viewed by others
References
Arcamone F., Cassinelli G., Ferni G., Penco S., Pennella P., Pol C.: Ergotamine production and metabolism ofClaviceps purpurea strain 275 FI in stirred fermenters.Can.J.Microbiol.16, 923–931 (1970).
Bandyopadhyay R., Frederickson D.E., McLaren N.W., Odvody G.N., Ryley M.J.: Ergot: a new disease threat to sorghum in the Americas and Australia.Plant Dis.82, 356–367 (1998).
Bhuiyan S.A., Galea V.J., Ryley M.J., Tay D., Lisle A.T.: Survival of conidia of sorghum ergot (caused byClaviceps africana) on panicles, seed and soil in Australia.Australas.Plant Pathol.31, 137–141 (2000).
Bogo A.: Biochemical physiopathology or some ergot fungi and other honeydew-producing plant parasites.PhD Thesis. Imperial College of Science, Technology and Medicine, London 2000.
Bogo A., Mantle P.G., Harthmann E.L.: Screening of sweet sorghum accessions for inhibition of secondary sporulation and saccharide measurements in honeydew ofClaviceps africana.Fitopatol.Bras.29, 86–90 (2004).
Bohnert H.J., Nelson D.E., Jensen R.G.: Adaptations to environmental stresses.Plant Cell7, 1099–1111 (1995).
Boon-Long T.: Sorghum diseases in Thailand, pp. 41–43 in W.A.J. de Milliano, R.A. Frederiksen, G.D. Bengston (Eds):Sorghum and Millet Diseases: 2nd World Review. International Crops Research Institute for the Semi-Arid Tropics, Patancheru (India) 1992.
Dickerson A.G.: A β-d-frcutofuranosidase fromClaviceps purpurea.Biochem.J.129, 263–272 (1972).
FAO: The World Sorghum and Millet Economies: Fact, Trends and Outlook. Food and Agriculture Organization of the United Nations, and International Crops Research Institute for the Semi-Arid Tropics, Rome (Italy) 1996.
Flieger M., Zelenkova N.F., Sedmera P., Novák J., Křen V., Rylko V., Sajdl P., Řeháček Z.: Ergot alkaloid glycosides from saprophytic cultures ofClaviceps — 1. Elymoclavine fructosides.J.Nat.Prod.52, 506–510 (1989).
Flieger M., Kantorová M., Pažoutová S., Kolínská R., Halada P., Stodůlková E., Sobotka M., Votruba J.: Physiological dendrogram ofClaviceps spp. based on sucrose metabolism in submerged cultures and its comparison with phylogenetic tree.Folia Microbiol.49, 705–712 (2004).
Frederickson D.E., Mantle P.G., de Milliano W.A.J.:Claviceps africana sp.nov.: the distinctive ergot pathogen of sorghum in Africa.Mycol.Res.95, 1101–1107 (1991).
Frederickson D.E., Mantle P.G., de Milliano W.A.J.: Windborne spread of ergot disease (Claviceps africana) in sorghum A-lines in Zimbabwe.Plant Pathol.42, 368–377 (1993).
Futrell M.C., Webster O.J.: Host range and epidemiology of the sorghum ergot organismPlant Dis.Rep.50, 828–831 (1966).
Havlíček V., Flieger M., Křen V., Ryska M.: Fast-atom-bombardment mass-spectrometry of elymoclavine glycosides.Biol.Mass Spectrom.23, 57–60 (1994).
Komolong B., Chakraborty S., Ryley M., Yates D.: Identity and genetic diversity of the sorghum ergot pathogen in Australia.Austral.J.Agric.Res.53, 621–628 (2002).
Kulkarni B.G.P., Seshadri V.S., Hegde R.K.: The perfect stage ofSphacelia sorghiMcRae.Mysore J.Agric.Sci.10, 286–289 (1976).
Mantle P.G., Bogo A.: Biosynthesis of bioactive honeydew oligosaccharides by sorghum ergot pathogens, pp. 91–94 in J.F. Leslie (Ed.):Sorghum and Millets Diseases, 2nd ed. Iowa State Press, Iowa City (USA) 2002.
McRae W.: Notes on some south Indian fungi.Madras Agric.Yearb. 108–111 (1917).
Mower R.L., Hancock J.G.: Sugar composition of ergot honeydews.Can.J.Bot.53, 2813–2825 (1975).
Pažoutová S., Frederickson D.E.: Genetic diversity ofClaviceps africana on sorghum andHyparrhenia. Plant Pathol.54, in press (2005).
Pažoutová S., Flieger M., Sajdl P., Řeháček Z., Taisinger J., Bass A.: The relationship between intensity of oxidative metabolism and predominance of agroclavine or elymoclavine in submergedClaviceps purpurea cultures.J.Nat.Prod.44, 225–236 (1981).
Pažoutová S., Bandyopadhyay R., Frederickson D.E., Mantle P.G., Frederiksen R.A.: Relations among sorghum ergot isolates from the Americas, Africa, India, and Australia.Plant Dis.84, 437–442 (2000).
Pažoutová S., Kolařík M., Kolínská R.: Pleomorphic conidiation inClaviceps.Mycol.Res.108, 126–135 (2004).
Pharr D.M., Stoop J.M.H., Williamson J.D., Studer-Feusi M.E., Massel M.O., Conkling M.A.: The dual role of mannitol as osmoprotectant and photoassimilate in celery.Hort.Sci.30, 1182–1188 (1995).
Reis E.M., Mantle P.G., Hassan H.A.G.: First report in the Americas ofSorghum ergot disease, caused by a pathogen diagnosed asClaviceps africana.Plant Dis.80, 463 (1996).
Tooley P.W., O’Neill N.R., Goley E.D., Carras M.M.: Assessment of diversity inClaviceps africana and otherClaviceps species by RAM and AFLP analyses.Phytopathology90, 1126–1130 (2000).
Tsukiboshi T., Shimanuki T., Uematsu T.:Claviceps sorghicola sp.nov., a destructive ergot pathogen of sorghum in Japan.Mycol.Res.103, 1403–1408 (1999).
Yancey P.H., Clark M.E., Hand S.C., Bowles R.D., Somero G.N.: Living with water-stress — evolution of osmolyte systems.Science217, 1214–1222 (1982).
Yun J.W.: Fructooligosaccharides — occurrence, preparation, and application.Enzyme Microb.Technol.19, 107–117 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
We express our appreciation to Prof. P.G. Mantle for his gift of honeydewC. sorghi. The research was supported by theGrant Agency of the Czech Republic (grant 525/00/1283),Universities Development Fund (grant 2052/2001), and byInstitutional Research Concept AV 0Z 5020 0510.
Rights and permissions
About this article
Cite this article
Flieger, M., Kantorová, M., Halada, P. et al. Oligosaccharides produced by submerged cultures ofClaviceps africana andClaviceps sorghi . Folia Microbiol 50, 198–204 (2005). https://doi.org/10.1007/BF02931566
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02931566