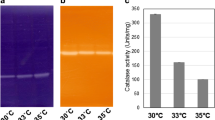
Abstract
Bacterial isolatesComamonas terrigena N3H (from soil contaminated with crude oil) andC. testosteroni (isolated from the sludge of a wastewater treatment plant), exhibit much higher total catalase activity than the same species from laboratory collection cultures. Electrophoretic resolution of catalases revealed only one corresponding band in cell-free extracts of bothC. testosteroni cultures. Isolates ofC. terrigena N3H exhibited catalase-1 and catalase-2 activity, whereas in the collection cultureC. terrigena ATCC 8461 only catalase-1 was detected. The environmental isolates exhibited much higher resistance to exogenous H2O2 (20, 40 mmol/L) than collection cultures, mainly in the middle and late exponential growth phases. The stepwise H2O2-adapted culture ofC. terrigena N3H, which was more resistant to oxidative stress than the original isolate, exhibited an increase of catalase and peroxidase activity represented by catalase-1. Pretreatment of cells with 0.5 mmol/L H2O2 followed by an application of the oxidative agent in toxic concentrations (up to 40 mmol/L) increased the rate of cell survival in the original isolate, but not in the H2O2-adapted variant. The protection of bacteria caused by such pretreatment corresponded with stimulation of catalase activity in pretreated culture.
Similar content being viewed by others
Abbreviations
- EEP:
-
early exponential phase
- LSP:
-
late stationary phase
- LEP:
-
late exponential phase
- MEP:
-
middle exponential phase
Referencés
Anderson A.J., Miller C.D.: Catalase activity and the survival ofPseudomonas putida, a root colonizer, upon treatment with peracetic acid.Can.J.Microbiol. 47, 222–228 (2001).
Bae H.S., Lee J.M., Kim Y.B., Lee S.T.: Biodegradation of the mixture of 4-chlorophenol and phenol byComamonas testosteroni CPW301.Biodegradation 7, 463–469 (1996).
Barkay T., Pritchard H.: Adaptation of aquatic microbial communities to pollutant stress.Microbiol.Sci. 5, 165–169 (1988).
Bishai W.R., Howard N.S., Winkelstein J.A., Smith H.O.: Characterization and virulence analysis of catalase mutants ofHaemophilus influenzae.Infect.Immun. 62, 4855–4860 (1994).
Bradford M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal.Biochem. 72, 248–250 (1976).
Dowds B.C.: The oxidative stress response inBacillus subtilis.FEMS Microbiol.Lett. 124, 255–263 (1994).
Dowds B.C.A., Murphy P., McConnell D.J., Devine K.M.: Relationship among oxidative stress, growth cycle, and sporulation inBacillus subtilis.J.Bacteriol. 169, 5771–5775 (1987).
Ferianc P., Polek B., Godočiková J., Tóth D.: Metabolic activity of cadmium-stressed and/or starvedVibrio sp.Folia Microbiol. 40, 443–446 (1995).
Gille G., Sigler K.: Oxidative stress and living cells.Folia Microbiol. 40, 131–152 (1995).
Godočiková J., Polek B., Whie G.F., Ferianc P., Tóth D.: Dioctyl sulphosuccinate utilization and mineralization by bacteriaComamonas terrigena N3H.Biologia 52, 741–745 (1997).
Godočíková J., Ferianc P., Polek B.: Lag period of14CO2 evolution from dioctyl sulpho[2,3-14C]succinate in relation to adaptation of bacterium,Comamonas terrigena, to dialkyl esters of sulphosuccinate.Biotechnol.Lett. 26, 1497–1500 (2004).
Halliwell B., Gutteridge M.C.:Free Radicals in Biology and Medicine, pp. 105–350. Oxford University Press, Oxford 1999.
Jenkins D.E., Schultz J.E., Matin A.: Starvation-induced cross-protection against heat and H2O2 challenge inEscherichia coli.J.Bacteriol. 170, 3910–3914 (1988).
Kappus H.: Oxidative stress in chemical toxicity.Arch.Toxicol. 60, 144–149 (1987).
Loprasert S., Vattanaviboon P., Praituan W., Chamnongpol S., Mongkolsuk S.: Regulation of the oxidative stress protective enzymes, catalase and superoxide dismutase inXanthomonas — a review.Gene 179, 33–37 (1996).
Muley M.R., Switala J., Borys A., Loewen P.C.: Regulation of transcription ofkatF inEscherichia coli.J.Bacteriol. 172, 6713–6720 (1990).
Murphy P., Dowds B.C.A., McConnel D.J., Devine K.M.: Oxidative stress and growth temperature inBacillus subtilis.J.Bacteriol. 169, 5766–5770 (1987).
Odenbreit S., Wieland B., Haas R.: Cloning and genetic characterization ofHelicobacter pylori catalase and construction of a catalase-deficient mutant strain.J.Bacteriol. 178, 6960–6967 (1996).
Park Y.B., Han Y.H.: Increased activities of SOD and catalases on aerobic growth inArcobacter nitrofigilis.J.Microbiol. 35, 239–240 (1997).
Peters M., Heinaru A., Nurk A.: Plasmid-encoded catalase KatA, the main catalase ofPseudomonas fluorescens strain Cb36.FEMS Microbiol.Lett. 200, 235–240 (2000).
Prokšová M., Augustín J., Vrbanová A.: Enrichment, isolation and characterization of dialkyl sulfosuccinate degrading bacteriaComamonas terrigena N3H andComamonas terrigena N1C.Folia Microbiol. 42, 635–639 (1997).
Roggenkamp R., Sahm H., Wagner F.: Microbial assimilation of methanol induction and function of catalase inCandida boidinii.FEBS Lett. 41, 283–286 (1974).
Sha Z., Stabel T.J., Mayfield J.E.:Brucella abortus catalase is a periplasmic protein lacking a standard signal sequence.J.Bacteriol. 176, 7375–7377 (1994).
Vattanaviboon P., Mongkolsuk S.: Unusual adaptive, cross protection responses and growth phase resistance against peroxide killing in a bacterial shrimp pathogen,Vibrio harveyi.FEMS Microbiol.Lett. 200, 111–116 (2001).
Vattanaviboon P., Whangsuk W., Mongkolsuk S.: A suppressor of the menadione-hypersensitive phenotype of aXanthomonas campestris pv. phascoli oxyR mutant reveals a novel mechanism of toxicity and the protective role of alkyl hydroperoxide reductase.J.Bacteriol. 185, 1743–1748 (2003).
Winquist L., Rannung U., Rannung A., Ramel C.: Protection from toxic and mutagenic effects of hydrogen peroxide by catalase induction inSalmonella typhimurium.Mutat.Res. 141, 145–147 (1984).
Woodbury W.A., Spencer K., Stahlmann M.A.: An improved procedure using ferricyanide for detecting catalase isozymes.Anal. Biochem. 44, 301–305 (1971).
Yumoto I., Iwata H., Sawabe T., Veno K., Ichise N., Matsujama H., Okuyama H., Kawasaki K.: Characterization of facultatively psychrophilic bacteriumVibrio rumoiensis sp.nov., that exhibits high catalase activity.Appl.Environ.Microbiol. 65, 67–72 (1999).
Yun E.J., Lee Y.N.: Production of two different catalase-peroxidases byDeinococcus radiophilus.FEMS Microbiol.Lett. 184, 155–159 (2000).
Zámocký M., Herzog C., Nykyri L.M., Koller F.: Site-directed mutagenesis of the lower parts of yeast catalase A leads to highly increased peroxidatic activity.FEBS Lett. 367, 241–245 (1995).
Zámocký M., Godočíková J., Koller F., Polek B.: Potential application of catalase-peroxidase fromComamonas terrigena N3H in the biodegradation of phenolic compounds.Antonie van Leeuwenhoek 79, 109–117 (2001).
Zámocký M., Polek B., Godočíková J., Koller F.: Oxidative stress-induced expression of catalases inComamonas terrigena.Folia Microbiol. 47, 235–240 (2002).
Zámocký M., Godočiková J., Gašperík J., Koller F., Polek B.: Expression, purification, and sequence analysis of catalase-1 from the soil bacteriumComamonas terrigena N3H.Protein Expres.Purif. 36, 115–123 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by VEGA grant of theSlovak Academy of Sciences no. 2/5069/25.
Rights and permissions
About this article
Cite this article
Godočíková, J., Boháčová, V., Zámocký, M. et al. Production of catalases byComamonas spp. and resistance to oxidative stress. Folia Microbiol 50, 113–118 (2005). https://doi.org/10.1007/BF02931458
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02931458