Abstract
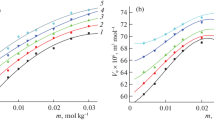
The activation and inactivation of adenylate kinase during deneturation in urea are compared with changes in UV absorbance at 287 nm. CD spectrum change at 222 nm, fluorescence intensity of ANS biding and small angle of X-ray scattering. At 1 mol/L, of urea the enzyme is activated 1.5-fold companied with a subtie decressing of its second structure, whereas its tertiary structure is fairly resistant to denaturation. By comparing the studies of the crystal structure and the mechanism of the catalysis of adenylste kinase, the activation is believed to result the effect that low concentration of urea increases the flexibility of the active site of the enzyme. This suggestion was confirmed by the results of the fluorescence intensity changes of ANS binding to adenylate kinase versus the concentration of urea.
Similar content being viewed by others
References
Zhang, Y. L., Zhou, J. M., Tsou, C. L., Inactivation precedes conformation change during thermal denaturation of adenylate kinase,Biochim. Biophys. Acta, 1993, 1164: 61
Zhang, Y. L., Zhou, J. M., Tsou, C. L., Sequential unfolding of adenylate kinase during denaturation by guanidine hydrochloride,Biochim. Biophys. Acta, 1996, 1295: 239.
Tsou, C. L., Inactivation precedes overall molecular conformation changes during enzyme denaturation.Biochim. Biophys. Acta, 1995, 1253: 151.
Glatter, O., Kratky, O.,Small-Angle X-ray Scattering, New York: Academic Press. 1982, 119.
Yao, Q. Z., Tian, M., Tsou, C. L., Comparison of the rates of inactivation and conformational changes of creatine kinase during urea denaturation,Biochemistry, 1984, 23: 2740.
Liu, W., Tsou, C. L., Activity change during unfolding of bovine pancreatic ribonuclease A in guenidine hydrochloride,Biochim. Biophys. Acta. 1987, 916: 455.
Xie, G. F., Tsou, C. L., Conformational and activity changes during guanidine deneturation of D-glyceraldehyde-3-phosphate dehydrogenase,Biochim, Biophys. Acta. 1987, 911: 19.
Tsou, C. L., Conformational flexibility of enzyme active sites,Science, 1993, 262: 380.
Gerstein, M., Schulz, G., Chorhia, C., Domain closure in adenylate kinase, joints on either side of two helices close like neighboring fingers,J. Mol. Biol., 1993, 229: 494.
Hamada, M., Kuby, S. A., Studies on adenosine triphospthate transphosphorylase XIII—kinetic properties of the crystalline rabbit muscle ATP-AMP transphosphorylase (adenylate kinase) and a comparison with the crystalline calf muscle and liver adenylate kinase,Arch. Biochem, Biophy., 1978, 190: 772.
Semistnov, G. V., Rdionova, N. A., Razgulyaev, O. I. et al., Study of the “molten globule” intermediate state in protein folding by a hydrophobic fluorescent probe,Biopolymers, 1991, 31: 119.
Creighton, T. E.,Protein Folding, New York: W. H. Freeman, 1992, 243–300.
Pai, E. F., Sachsenheimer, W., Schirmer, R. H. et al., Substrate positions and induced-fit in cryatalline adenylate kinese.J. Mol. Biol., 1977, 114: 37.
Muller, C. W., Schulz, G. E., Structure ol the complex between adenylate kinase fromEsrherichia coli and the inhibitor Ap5A refined at 1.9 Å resolution,J. Mol. Biol., 1992, 224: 159.
Fan, Y. X., Ju, M., Zhou, J. M. et al., Activation nf chicken liver dihydrofolate reductase in concentrated urea solutions.Biochim. Biophs. Acta, 1995, 1252: 151.
Author information
Authors and Affiliations
Additional information
Project partly supported by the National Climbing Programme of China.
Rights and permissions
About this article
Cite this article
Zhang, H., Pan, X., Zhou, J. et al. Activation and conformational changes of adenylate kinase in urea solution. Sci. China Ser. C.-Life Sci. 41, 245–250 (1998). https://doi.org/10.1007/BF02895098
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02895098