Abstract
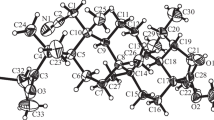
A new method for the preparation of the synthon (±)-2,6,7,7a-tetrahydro-1β-hydroxy-4-formyl-7aβ-methylindene (1,a) for the total synthesis of steroids in both (±) and (+) forms, starting from the known β-ketoester, (±)-methyl 1β-t-butoxy-5,6,7,7a-tetrahydro-7aβ-methyl-5-keto-4-indancarboxylate (2,a) has been described. An alternative route to (1,a) has been investigated. Although the compound, (±)-1β-hydroxy-5,6,7,7a-tetrahydro-7aβ-methyl-5-keto-4-methoxymethylindan (2,b) could not be prepared, interesting pathways leading to two unexpected products, (±)-5,6,7,7a-tetrahydro-4,7a-dimethyl-5H-indene-1,5-dione and (±)-2,6-diketo-3-methyltricyclo-(5,2,1,0)decan-8-ol (3 and 4), were encountered during an attempted annelation reaction of the ketone, N-diethylamino-5-methoxypentan-3-one (6), with 2-methylcyclopentan-1,3-dione (5). Trapping of the intermediate, (±)-3a,4,5,6,7,7a-hexahydro-3a-hydroxy-4-methylene-7a-methylindene-1,5-dione (7), through the formation of the adduct, (±)-3a,4,5,6,7,7a-hexahydro-3a-hydroxy-4-(1′, 3′-diketo-2′-methylcyclopentano-2′-methylene)-7a-methylindene-1,5-dione (8), established the mechanism of the formation of the products (3 and 4).
Similar content being viewed by others

References
Attenburrow J, Chapman J H, Evans R M, Hems B A, Jansen A B A and Pickles W 1952J. Chem. Soc. 1094
Baddeley G, Taylor H T and Pickles W 1953J. Chem. Soc. 124
Baggiolini E G, Iacobelli J A, Hennessy B H and Uskovic M R 1982J. Am. Chem. Soc. 104 2945
Balasubrahmanyam S N and Balasubramanian M 1973Org. Syn. Coll. 5 439
Banerjee D K, Vittal Rao A S, Venkataramu S D, Surendranath V and Angadi V B 1976Synthesis 307
Banerjee D K, Kasturi T R and Sarkar A 1983Proc. Indian Acad. Sci. (Chem. Sci.) 92 181
Bates E B, Jones E R H and Whiting M C 1954J. Chem. Soc. 1854
Boyce C B C and Whitehurst J S 1959J. Chem. Soc. 2022
Collins D J and Tomkins C W 1977Aust. J. Chem. 30 443
Danishefsky S and Migdalof B H 1969Tetrahedron Lett. 4331
Dorrow A, Messwarb G and Frey H H 1950Chem. Ber. 83 495
Ellis J E, Dutcher J S and Heathcock C H 1974Syn. Commun. 4 71
Hajos Z G and Parrish D R 1985Org. Syn. 63 26
Hochstein F A 1949J. Am. Chem. Soc. 71 305
Hochstein F A and Brown W G 1948J. Am. Chem. Soc. 70 3484
Kuo C H, Taub D and Wender N L 1965Angew. Chem. 77 1142
Mancuso A J, Huang S L and Swern D 1978J. Org. Chem. 43 2480
McCusker P A and Kroeger J W 1937J. Am. Chem. Soc. 59 214
Micheli R A, Hajos Z G, Cohen N, Parrish D R, Portland L A, Sciamanna W, Scott M A and Wehrli P A 1975J. Org. Chem. 40 675
Nystorn R F and Brown W G 1947J. Am. Chem. Soc. 69 2548
Zoretic P A, Bendiksen B and Branchand B 1976J. Org. Chem. 41 3767
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saibaba, R., Banerjee, D.K., Kasturi, T.R. et al. A new synthesis of (±) and (+)-2, 6, 7, 7a-tetrahydro-1β-hydroxy-4-formyl-7aβ-methylindenes. Proc. Indian Acad. Sci. (Chem. Sci.) 99, 327–339 (1987). https://doi.org/10.1007/BF02880456
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02880456