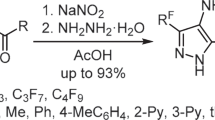
Abstract
2-Amino-2-arylethylamides1 carrying electron-donating substituents in thepara position are transformed by hot POC13 to the the title compounds2, presumably via iminochlorides 7 and imidazolium derivatives8. Amides lacking this para-substituent give rise to chloroamidines11 under these conditions.m-Methoxyphenethylamide1t and POCl3 form, besides11f, an isoquinoline derivative3. The involvement of an imidazolium compound8 in the formation of ethenamidines has been verified by the synthesis of2a from10. Reaction of amide1w with PCl5 in the cold leads to, besides the chloroamidine11c, thecis-ethenamidine12 which equilibrates with thetrans-isomer2o in hot toluene. Thienylethyl urea13 converted by hot POCl3 to the imidazoline16, while phenylpropylamide17 forms only the iminochloride18a.
Similar content being viewed by others
References
Advani B G, Nagarajan K, Rajagopalan P and Ranga Rao V 1968Tetrahedron Lett. 56 5825
Biere H 1983Eur. Patent ammeldung EP 110 090A
Compagnon P L, Gasquez F, Compagnon O and Kimny T 1982Bull. Soc. Chim. Belg. 91 931
Cook S L, Venayak N D and Wakefield B J 1983J. Chem. Res. Synp. 199
Cook S L and Wakefield B J 1980J. Chem. Soc., Perkin Trans. I. 2392
Ichimura H and Ohta M 1966Tetrahedron Lett. 54 807
Islam A M, El-Sharief A M S, Ismail I M and Harb A A 1983Egypt J. Chem. 26 221; 1984Chem. Abstr. 101 191751g
Ito M M, Nomura Y, Takeuchi Y and Tomoda S 1983Bull. Chem. Soc. Jpn. 56 641
Kantlehner W 1979Advances in organic chemistry methods and results (series ed.) E C Taylor. Vol. 9, Part 2.Iminium salts in organic chemistry (eds) H Bohme and H G Viehe (New York: John Wiley & Sons) p.99
Marxer A 1972Helv. Chim. Acta 55 430
Matier W L and Corner W T 1974J. Org. Chem. 39 3080
Matier W L and Corner W T 1975Ger. Offen DE 2428223; 1975Chem. Abstr. 82 170425c
Matier W L and Corner W T 1977Ger. Offen. DE 2635121; 1977Chem. Abstr. 87 22805q
McKillop A and Boulton A J (eds) 1984Comprehensive heterocyclic chemistry (Oxford: Pergamon) vol. 2, p. 442
Nagarajan K, Vunnam V R, Shah R K, Shenoy S J, Fritz H, Richter W J and Muller D 1988Helv. Chim. Acta 71 77
Rajagopalan P and Advani B G 1965Tetrahedron Lett. 26 2197
Ried W and Erie H E 1979Chem. Ber. 112 640
Va Meeteren H W and Van der Plas H C 1971Recl. Trav. Chim. Pays-Bas 90 105
Yanagida S 1975Jpn. Patent 50/37671; 1976Chem. Abstr. 85 108660j
Yanagida S, Fujita T, Ohoka M, Katagiri I and Komori S 1973Bull. Chem. Soc. Jpn. 46 292
Author information
Authors and Affiliations
Additional information
Contribution No. 752 from Research Centre
Rights and permissions
About this article
Cite this article
Nagarajan, K., Rajagopalan, P., Advani, B.G. et al. Synthesis oftrans-N-2-aryl(heteryl)ethenamidines. Proc. Indian Acad. Sci. (Chem. Sci.) 104, 383–397 (1992). https://doi.org/10.1007/BF02839548
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02839548