Abstract
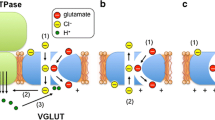
Recently, we isolated a novel subcellular fraction of glial plasmalemmal vesicles (GPV), which showed a higher activity of Na+-dependent glutamate transport than synaptosomes (Nakamura et al., 1993). In order to study kinetically the glutamate transport mechanism, we measured the reaction under various ionic conditions both inside and outside the vesicles. The vesicles treated hypotonically and preloaded with KCl could take up glutamate in the presence of external Na+. The level of glutamate uptake was dependent on external concentrations of NaCl ([NaCl]o) and competitively inhibited by [KCl]o. However, it was dependent on [KCl]i, and competitively inhibited by [NaCl]i. The activation and inhibition constants of K+ were about 30 mM inside and 20 mM outside, respectively, whereas those of Na+ were 140 mM outside and 4 mM inside, respectively. These results suggest that the transport carrier molecules work asymmetrically to the membranes. Nigericin and monensin, acidic ionophores for K+ and Na+, respectively, inhibited the glutamate uptake. On the other hand, valinomycin, a neutral ionophore for K+, elevated the uptake level, suggesting that the inside-negative membrane potential induced by K+ diffusion enhances the uptake activity. We conclude that glutamate transport by glial cells requires both external Na+ and internal K+ and is regulated by the membrane potential.
Similar content being viewed by others
References
Barbour B., Brew H., and Attwell D. (1988) Electrogenic glutamate uptake in glial cells is activated by intracellular potassium.Nature 335, 433–435.
Benveniste H., Drejer J., Schousboe A., and Dieme N. H. (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebralmicrodialysis.J. Neurochem. 43, 1369–1374.
Drejer J., Larsson O. M., and Schousboe A. (1982) Characterization of L-glutamate uptake and release from astrocytes and neurons cultured from different brain regions.Exp Brain Res. 47, 259–269.
Kanai Y. and Hediger M. A. (1992) Primary structure and functional characterization of a high-affinity glutamate transporter.Nature 360, 467–471.
Kanner B. I. (1978) Active transport of γ-aminobutyric acid by membrane vesicles isolated from rat brain.Biochemistry 17, 1207–1211.
Kanner B. I. (1983) Bioenergetics of neurotransmitter transport.Biochim. Biophys. Acta 726, 293–316.
Kanner B. I. and Schuldiner S. (1987) Mechanism of transport and storage of neurotransmitters.CRC Crit. Rev. Biochem. 22, 1–38.
Kanner B. I. and Sharon I. (1978) Active transport of L-glutamate by membrane vesicles isolated from rat brain.Biochemistry 17, 3949–3953.
Mitani A., Kubo H., Iga K., Imon H., Kadoya F., and Kataoka K. (1990) A new enzymatic cycling technique for glutamate determination in brain microdialysates.J. Neurochem. 54, 709–711.
Nakamura Y., Iga K., Shibata T., Shudo M., and Kataoka K. (1993) Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes.Glia 9, 48–56.
Ogata T., Nakamura Y., Shibata T., and Kataoka K. (1992) Release of excitatory amino acids from cultured hippocampal astrocytes induced by a hypoxic-hypoglycemic stimulation.J. Neurochem. 58, 1957–1959.
Pines G., Danbolt N. C., Bjoras M., Zhang Y., Bendahan A., Eide L., Koepsell H., Storm-Mathiesen J., Seeberg E., and Kanner B. I. (1992) Cloning and expression of a rat brain L-glutamate transporter.Nature 360, 464–467.
Schousboe A., Drejer J., and Hertz L. (1988) Uptake and release of glutamate and glutamine in neurons and astrocytes in primary cultures, inGlutamine and Glutamate in Mammals, (Kvamme E., ed.), CRC, Boca Raton, FL, pp. 21–38.
Storck T., Schulte S., Hofmann K., and Stoffel W. (1992) Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain.Proc. Natl. Acad. Sci. USA 89, 10,955–10,959.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakamura, Y., Kataoka, K. Transport mechanism of glutamate by hypotonic-treated glial plasmalemmal vesicles from rat hippocampus. J Mol Neurosci 4, 255–262 (1993). https://doi.org/10.1007/BF02821557
Issue Date:
DOI: https://doi.org/10.1007/BF02821557