Abstract
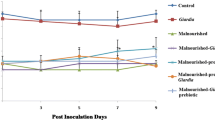
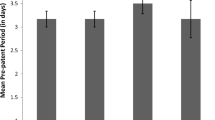
The influence of acute dietary protein restriction on the development ofBabesia microti infection in the mouse model was investigated. Female mice consuming a diet either devoid of protein or adequate with respect to protein were infected withB. microti-parasitized erythrocytes and sacrificed 7 days later. Absence of dietary protein resulted in a delay in the onset of infection and a significantly reduced peak parasitemia. Non-specific antibody responses to heterologous erythrocytes and specific anti-babesial antibody titers were impaired in mice consuming the protein-free diets, suggesting that the enhanced resistance to experimental babesiosis observed in protein-malnourished mice is not an antibodymediated phenomenon. In addition, protein-malnourished mice did not demonstrate significantly lower concentrations of the serum complement component, C3, which has been implicated as a participant in the invasion process of host erythrocytes by parasites. Serum C3 levels were significantly reduced in infected mice consuming both diets. The mechanism by which acute protein deprivation protects mice against lethal babesiosis remains to be determined.
Similar content being viewed by others
References
Ajayi S, Wilson A, Campbell R (1978) Experimental bovine anaplasmosis: clinico-pathological and nutritional status. Res Vet Sci 25:76–81
Beisel W (1982) Synergism and antagonism of parasitic diseases and malnutrition. Rev Infect Dis 4:746–750
Benach J, Habicht G, Hamburger M (1982) Immunoresponsiveness in acute babesiosis. J Infect Dis 146:369–380
Callow L, Dalgliesh R (1982) Immunity and immunopathology in babesiosis. In: Cohen S, Warren K (eds) Immunology of parasitic infections. Blackwell Scientific, Oxford, pp 475–526
Carlomagno M, McMurray D (1983) Chronic zinc deficiency in rats: its influence on some parameters of humoral and cell-mediated immunity. Nutr Res 3:69–78
Carlomagno M, Alito A, Almiron D, Gimeno A (1982) T and B lymphocyte function in response to a protein-free diet. Infect Immun 38:195–200
Chapman W, Ward P (1976) The complement profile in babesiosis. J Immunol 117:935–938
Cooper L, Good R, Mariani T (1974) Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr 27:647–664
Curnow J (1968) In-vitro agglutination of bovine erythrocytes infected withBabesia argentina. Nature 217:267–268
Edirisinghe J, Fern E, Targett G (1981) The influence of dietary protein on the development of malaria. Ann Trop Pediatr 1:87–91
Edirisinghe J, Fern E, Targett G (1981) Dietary suppression of rodent malaria. Trans R Soc Trop Med Hyg 75:591–593
Fern E, Edirisinghe J, Targett G (1984) Increased severity of malaria infection in rats fed supplementary amino acids. Trans R Soc Trop Med Hyg 78:839–841
Gray G, Phillips R (1981) Use of sorbitol in the cryopreservation of babesia. Res Vet Sci 30:388–389
Gray G, Phillips R (1983) Suppression of primary and secondary antibody responses and inhibition of antigen priming duringBabesia microti infections in mice. Parasitol Immunol 5:123–134
Hussein H (1976)Babesia hylomysci in mice: preference for erythrocytes of a particular age-group and pathogenesis of the anaemia. Z Parasitenk 50:103–108
Jack R, Ward P (1981) The entry process of babesia merozoites into red cells. Am J Pathol 102:109–113
James M, Kuttler K, Levy M, Ristic (1981) Antibody kinetics in response to vaccination againstBabesia bovis. Am J Vet Res 42:1999–2001
Jerne N, Nordin A, Henry C (1963) The agar plaque technique for recognizing antibody-producing cells. In: Amos B, Koprowski H (eds) Cell-bound antibodies. Wistar Institute Press, Philadelphia, pp 109–125
Lachman P, Hobart M (1978) Complement technology. In: Weir D (ed) Handbook of experimental immunology I, 3rd edn. Blackwell Scientific, Oxford
Latif B, Said M, Ali S (1979) Effect of age on the immune response of cattle experimentally infected withBabesia bigemina. Vet Parasitol 5:307–314
Levy M, Clabaugh G, Ristic M (1982) Age resistance in bovine babesiosis: role of blood factors in resistance toBabesia bovis. Infect Immun 37:1127–1131
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurements with the Folinphenol reagent. J Biol Chem 193:265–275
Mancini G, Carbonara A, Heremans J (1965) Immunochemical quantitation of antigens by single radial immunodiffusion. J Immunochem 2:235–254
Meeusen E, Lloyd S, Soulsby EJL (1984)Babesia microti in mice. Subpopulations of cells involved in the adoptive transfer for immunity with immune spleen cells. Aust J Exp Biol Med Sci 63:567–575
National Academy of Science — National Research Council (1963) Evaluation of protein quality, 1100. National Academy of Science, Washington, DC, pp 46–55
Phillips R (1969) The protective activity of serum from immune rats againstBabesia rodhaini. Parasitology 59:357–364
Rogers R (1974) Serum opsonins and the passive transfer of protection inBabesia rodhaini infections of rats. Int J Parasitol 4:197–201
Rosner F, Zarrabi M, Benach J, Habicht G (1984) Babesiosis in splenectomized adults. Am J Med 76:696–701
Ruebush M, Hanson W (1979) Susceptibility of five strains of mice toBabesia microti of human origin. J Parasitol 65:430–433
Scrimshaw N, Taylor C, Gordon J (1968) Interactions of nutrition and infection. Monograph series 57. WHO, Geneva
Suskind R (1977) Malnutrition and the immune response. Raven Press, New York
Watson R, McMurray D (1979) The effects of malnutrition on secretory and cellular immune processes. Crit Rev Food Sci Nutr 12:113–159
Wilson A, Trueman K (1978) Some effects of reduced energy intake on the development of anaplasmosis inBos indicus cross steers. Aust Vet J 54:121–124
Wolf R (1974) Effects of antilymphocyte serum and splenectomy on resistance toBabesia microti infection on hamsters. Clin Immunol Immunopathol 2:381–394
Author information
Authors and Affiliations
Additional information
Presented, in part, at the 1984 meeting of the Federation of American Societies for Experimental Biology, St. Louis, Missouri, USA
Rights and permissions
About this article
Cite this article
Tetzlaff, C.L., Carlomagno, M.A. & McMurray, D.N. Reduced dietary protein content suppresses infection withBabesia microti . Med Microbiol Immunol 177, 305–315 (1988). https://doi.org/10.1007/BF02389902
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02389902