Abstract
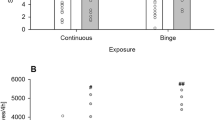
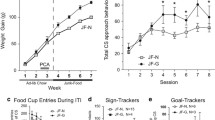
Rats exhibit profound individual differences in their propensity to ingest sugar and in their locomotor response to AMP. Intrinsic variation in the responsiveness of mesolimbic dopamine mechanisms has been suggested to account for these individual differences. In light of this overlap, it might be expected that individual differences in one behavior would predict individual differences in the other. The present study determined whether individual differences in sugar intake would predict individual differences in the locomotor response to AMP. Male Wistar rats were divided into low and high feeders based on a median split of their sugar intake in response to saline administration and were subsequently tested for their locomotor response to either 1.0 or 1.75 mg/kg AMP in experiment 1. High sugar feeders exhibited significantly more locomotion than low sugar feeders in response to 1.75 mg/kg AMP. This difference was observed immediately after injection and continued for approximately 90 min. There was no difference between the two groups in their locomotor response to 1.0 mg/kg AMP. In experiment 2, rats receiving 1.0 mg/kg AMP in experiment 1 were tested for the development of behavioral sensitization with repeated AMP administrations. Rats were administered 1.0 mg/kg AMP across 5 test days, interspersed with days in which they received AMP treatment in their home cages to minimize conditioning effects. High sugar feeders exhibited greater behavioral sensitization than low sugar feeders with repeated AMP administration. Starting on test day 3, high sugar feeders exhibited significantly greater AMP-induced locomotor activity than low sugar feeders. Taken together, these findings support the notion that there is overlap in the neurobiological substrates regulating sugar intake and responsivity to the locomotor activating effect of AMP. Furthermore, these results establish that the propensity to ingest sugar is a predictor of the susceptibility to the locomotor enhancing properties of AMP.
Similar content being viewed by others
References
Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52: 1655–1658
Antelman SM, Szechtman H (1975) Tail-pinch induces eating in sated rats which appears to depend on nigrostriatal dopamine. Science 189: 731–733
Carr GD, White NM (1986) Contributions of dopamine terminal areas to amphetamine-induced anorexia and adipsia. Pharmacol Biochem Behav 25: 17–22
Cole BJ, Koob GF (1989) Low doses of corticotropin-releasing factor potentiate amphetamine-induced stereotyped behavior. Psychopharmacology 99: 27–33
Cole BJ, Cador M, Stinus L, Rivier C, Rivier J, Vale W, Le Moal M, Koob GF (1990a) Critical role of the hypothalamic pituitary adrenal axis in amphetamine-induced sensitization of behavior. Life Sci 47: 1715–1720
Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M (1990b) Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res 512: 343–346
Colle L, Wise RA (1988) Concurrent facilitory and inhibitory effects of amphetamine on stimulation-induced eating. Brain Res 459: 356–360
DiLullo SL, Martin-Iverson MT (1992) Evidence for presynaptic dopamine mechanisms underlying amphetamine-conditioned locomotion. Brain Res 578: 161–167
Eichler AJ, Antelman SM (1979) Sensitization to amphetamine and stress may involve nucleus accumbens and medial frontal cortex. Brain Res 176: 412–416
Evans KR, Vaccarino FJ (1986) Intra-nucleus accumbens amphetamine: dose-dependent effects on food intake. Pharmacol Biochem Behav 25: 1149–1151
Evans KR, Vaccarino FJ (1990) Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev 14: 9–22
Glickman SE, Schiff BB (1967) A biological theory of reinforcement. Psychol Rev 74: 81–109
Gold LH, Swerdlow NR, Koob GF (1988) The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci 102: 544–552
Heffner TG, Zigmond MJ, Stricker EM (1977) Effects of dopaminergic agonists and antagonists on feeding in intact and 6-hydroxydopamine-treated rats. J Pharmacol Exp Ther 201: 386–399
Herman J-P, Stinus L, Le Moal M (1984) Repeated stress increases locomotor response to amphetamine. Psychopharmacology 84: 431–435
Hernandez L, Hoebel BG (1988a) Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav 44: 599–606
Hernandez L, Hoebel BG (1988b) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42: 1705–1712
Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB, Jr (1991a) Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav 38: 467–470
Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB, Jr (1991b) Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse 9: 121–128
Hooks MS, Jones GH, Liem BJ, Justice JB, Jr (1992a) Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav 43: 815–823
Hooks MS, Jones GH, Neill DB, Justice JB, Jr (1992b) Individual differences in amphetamine sensitization: dose-dependent effects. Pharmacol Biochem Behav 41: 203–210
Imperato A, Puglisi-Allegra S, Casolinin P, Zocchi A, Angelucci L (1989) Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol 165: 337–338
Jones GH, Robbins TW (1992) Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav 43: 887–895
Jones GH, Marsden CA, Robbins TW (1990) Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology 102: 364–372
Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW (1992) Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav 43: 17–35
Joyce EM, Koob GF (1981) Amphetamine-, scopolamine- and caffeine-induced locomotor activity following 6-hydroxydopamine lesions of the mesolimbic dopamine systems. Psychopharmacology 73: 311–313
Kalivas PW, Duffy P (1989) Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry 25: 913–928
Kelly PH, Seviour PW, Iversen SD (1975) Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res 94: 507–522
Kuczenski R, Segal DS, Aizenstein ML (1991) Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci 11: 2703–2712
Leith NJ, Kuczenski R (1982) Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology 76: 310–315
Leyton M, Stewart J (1990) Preexposure to foot-shock sensitizes the locomotor response to subsequent systemic morphine and intra-nucleus accumbens amphetamine. Pharmacol Biochem Behav 37: 303–310
Maccari S, Piazza PV, Deminiere JM, Angelucci L, Simon H, Le Moal M (1991) Hippocampal type I and type II corticosteroid receptor affinities are reduced in rats predisposed to develop amphetamine self-administration. Brain Res 548: 305–309
Martin-Iverson MT, McManus DJ (1990) Stimulant-conditioned locomotion is not affected by blockade of D1 and/or D2 dopamine receptors during conditioning. Brain Res 521: 175–184
Mittleman G, Castaneda E, Robinson TE, Valenstin ES (1986) The propensity for nonregulatory ingestive behavior is related to differences in dopamine systems: behavioral and biochemical evidence. Behav Neurosci 100: 213–220
Morley JE, Levine AS, Rowland NE (1983) Stress induced eating. Life Sci 32: 2169–2182
Patrick SL, Thompson TL, Walker JM, Patrick RL (1991) Concomitant sensitization of amphetamine-induced behavioral stimulation and in vivo dopamine release from rat caudate nucleus. Brain Res 538: 343–346
Piazza PV, Deminiere JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245: 1511–1513
Piazza PV, Deminiere JM, Le Moal M, Simon H (1990a) Stress and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514: 22–26
Piazza PV, Rogue-Pont F, Deminiere JM, Kharouby M, Le Moal M, Simon H (1990b) Individual vulnerability to amphetamine self-administration is correlated with dopaminergic activity in frontal cortex and nucleus accumbens. Soc Neurosci Absr 16: 243.14
Piazza PV, Maccari S, Deminiere J-M, Le Moal M, Mormede P, Simon H (1991a) Corticosterone levels determine individual vulnerability to amphetamine self-administration. Pro Natl Acad Sci 88: 2088–2092
Piazza PV, Rogue-Pont F, Deminiere JM, Kharouby M, Le Moal M, Simon H (1991b) Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res 567: 169–174
Pijnenburg AJJ, Honig WMM, Van Rossum JM (1975) Inhibition ofd-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia 41: 87–95
Radhakishun FS, van Ree JM, Westerink BHC (1988) Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett 35: 351–356
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev 11: 157–198
Robinson TE, Becker JB, Young EA, Akil H, Castaneda E (1987) The effects of footshock stress on regional brain dopamine metabolism and pituitaryβ-endorphin release in rats previously sensitized to amphetamine. Neuropharmacology 26: 679–691
Robinson TE, Jurson PA, Bennett JA, Bentgen KM (1988) Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+) -amphetamine: a microdialysis study in freely moving rats. Brain Res 462: 211–222
Rowland NE, Antelman SM (1976) Stress-induced hyperphagia and obesity in rats: a possible model for understanding human obesity. Science 191: 310–312
Segal DS, Kuczenski R (1987) Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther 242: 917–926
Segal DS, Mandell AJ (1974) Long-term administration of amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 2: 249–255
Segal DS, Kelly P, Koob GF, Roberts DCS (1979) Non-striatal dopamine mechanisms in the response to repeated d-amphetamine administration. In: Usdin E, Kopin IJ, Barchas J (eds) Catecholamine: basic and clinical frontiers, Vol. 2. Pergamon, New York, pp 1672–1674
Souquet A-M, Rowland NE (1989) Effect of chronic administration of dexfenfluramine on stress-and palatability-induced food intake in rats. Physiol Behav 46: 145–149
Sills TL, Vaccarino FJ (1991) Facilitation and inhibition of feeding by a single dose of amphetamine: relationship to baseline intake and accumbens cholecystokinin. Psychopharmacology 105: 329–334
Sills TL, Baird JP, Vaccarino FJ (1993) Individual differences in the feeding effects of amphetamine: role of nucleus accumbens dopamine and circadian factors. Psychopharmacology 112: 233–241
Thierry AM, Tassin JP, Blanc G, Glowinski J (1976) Selective activation of the mesocortical DA system by stress. Nature 263: 242–243
Vaccarino FJ, Amalric M, Swerdlow NR, Koob GF (1986) Blockade of amphetamine but not opiate-induced locomotion following antagonism of dopamine function in the rat. Pharmacol Biochem Behav 24: 61–65
Vaccarino FJ, Schiff BB, Glickman SE (1989) Biological view of reinforcement. In: Klein SB, Mowrer RR (eds) Contemporary learning theories. Lawrence Erlbaum, New Jersey, pp 111–142
Vezina P, Stewart J (1989) The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res 499: 108–120
Wallach MB, Dawber M, McMahon M, Rogers C (1977) A new anorexigen assay: stress-induced hyperphagia in rats. Pharmacol Biochem Behav 6: 529–531
Weissenborn R, Winn P (1992) Regulatory behaviour, exploration and locomotion following NMDA or 6-OHDA lesions in the rat nucleus accumbens. Behav Brain Res 51: 127–137
Whishaw IQ, Fiorino D, Mittleman G, Castaneda E (1992) Do forebrain structures compete for behavioral expression? Evidence from amphetamine-induced behavior, microdialysis, and caudate-accumbens lesions in medial frontal cortex damaged rats. Brain Res 576: 1–11
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94: 469–492
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sills, T.L., Vaccarino, F.J. Individual differences in sugar intake predict the locomotor response to acute and repeated amphetamine administration. Psychopharmacology 116, 1–8 (1994). https://doi.org/10.1007/BF02244864
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02244864