Abstract
Three species of microalgae commonly used in mariculture —Isochrysis sp. (clone T.ISO) Parke,Pavlova lutheri (Droop) Green andNannochloropsis oculata (Droop) Green — were grown in batch and semicontinuous modes to compare their biochemical composition and production rates.
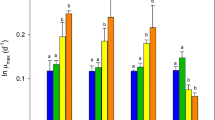
In batch mode, logarithmic-phase cultures of all species had high levels of protein (25.2 to 41.1‰) and low levels of carbohydrate (7.1 to 10.3‰) and lipid (8.8 to 14.9‰). At stationary phase, cultures ofIsochrysis sp. (clone T.ISO) andN. oculata contained significantly less protein (21.8‰ and 20.3‰, respectively), all species contained more carbohydrate (14.8 to 30.6‰), andP. lutheri contained more lipid (16.6‰). In semi-continuous mode, cultures maintained at late logarithmic-phase contained more carbohydrate,Isochrysis sp. (clone T.ISO) contained less protein, andP. lutheri more lipid than logarithmic-phase batch cultures of the same species. Neither growth phase nor harvest regime affected the amino acid composition of the microalgae significantly. However, the concentration of proline inN. oculata was higher in batch cultures in logarithmic phase (9.4‰), than in either semi-continuous cultures in logarithmic phase (5.8 to 7.9‰) or batch cultures in stationary phase (5.6 to 5.9‰).
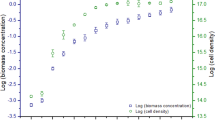
The production rates from batch and semi-continuous logarithmic-phase cultures were not significantly different for any of the species, and there were only minor differences in the production rates of the species (range 12.4 to 17.1 mg algae dry weight 1−1 d−1). The different culture and harvest regimes produced significant differences in the proportions of protein and carbohydrate in the microalgae. Which regime is chosen for culturing these microalgae as food will depend on the nutritional requirements of the animal species being fed.
Similar content being viewed by others
References
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917.
Brown MR (1991) The amino acid and sugar composition of sixteen species of microalgae used in mariculture. J. exp. mar. Biol. Ecol. 145: 79–99.
Brown MR, Jeffrey SW, Garland CD (1989) Nutritional aspects of microalgae used in mariculture; a literature review. CSIRO Marine Laboratories Report 205, 44 pp.
Caron L, Mortain-Bertrand A, Jupin H (1988) Effect of photoperiod on photosynthetic characteristics of two marine diatoms. J. exp. mar. Biol. Ecol. 123: 211–226.
Chau YK, Chuecas L, Riley AJP (1967) The component combined amino acids of some marine phytoplankton species. J. mar. biol. Ass. UK 47: 543–544.
Cole JJ (1982) Interactions between bacteria and algae in aquatic ecosystems. Ann. Rev. Ecol. Syst. 13: 291–314.
Dubois M, Gillies KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28: 350–356.
Dunstan GA, Volkman JK, Jeffrey SW, Barrett SM (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae 2. Lipids. J. exp. mar. Biol. Ecol., 161: 115–134.
Dunstan GA, Volkman JK, Barrett SM, Garland CD (1993) Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J. appl. Phycol. 5: 71–83.
Enright CT, Newkirk GF, Craigie JS, Castell JD (1986). Growth of juvenileOstrea edulis L. fedChaetoceros gracilis Schütt of varied chemical composition. J. exp. mar. Biol. 96: 14–25.
Fernández-Reiriz MJ, Perez-Camacho A, Ferreiro MJ, Blanco J, Phanas M, Campos MJ, Labarta U (1989) Biomass production and variation in the biochemical profile (total protein, carbohydrates, RNA, lipids and fatty acids) of seven species of marine microalgae. Aquaculture 83: 17–37.
Guillard RRL, Ryther JH (1962) Studies on marine planktonic diatoms. I.Cyclotella nana Hustedt, andDetonula confervacea (Cleve) Gran. Can. J. Microbiol. 8: 229–239.
Harrison PJ, Thompson PA, Calderwood GS (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J. appl. Phycol. 2: 45–56.
James CM, Al-Hinty S, Salman AE (1989) Growth and ω3 fatty acid and amino acid composition of microalgae under different temperature regimes. Aquaculture 77: 337–357.
Jeffrey SW (1980) Cultivating uni-cellular marine plants. CSIRO Division of Fisheries and Oceanography Annual Report, 1977–79, pp. 22–43.
Jeffrey SW, Garland CD, Brown MR (1989). Microalgae in Australian Mariculture. In Clayton MN & King RJ (eds), Marine Botany, Longman-Cheshire, Melbourne, pp. 400–414.
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophyllsa, b, c 1 andc 2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167: 191–194.
Redalje DG, Laws EA (1983) The effects of environmental factors on growth and the chemical and biochemical composition of marine diatoms. 1. Light and temperature effects. J. exp. mar. Biol. Ecol. 68: 59–79.
Teshima S, Kanazawa A (1984) Effects of protein, lipid, and carbohydrate levels in purified diets on growth and survival of the prawn larvae. Bull. Jpn. Soc. sci. Fish. 50: 1709–1715.
Thomas WH, Seibert DLR, Alden M, Eldridge P, Neori A (1984a) Yields, photosynthetic efficiencies and proximate composition of dense marine microalgal cultures. I. Introduction andPhaeodactylum tricornutum experiments. Biomass 5: 181–209.
Thomas WH, Seibert DLR, Alden M, Neori A, Eldridge P (1984b) Yields, photosynthetic efficiencies and proximate composition of dense marine microalgal cultures. II.Dunaliella primolecta andTetraselmis suecica experiments. Biomass 5: 211–225.
Thomas WH, Seibert DLR, Alden M, Neori A, Eldridge P (1984c). Yields, photosynthetic efficiencies and proximate composition of dense marine microalgal cultures. III.Isochrysis sp. andMonallantus salina experiments and comparative conclusions. Biomass 5: 299–316.
Thompson PA, Harrison PJ, Whyte JNC (1990) Influence of irradiance on the fatty acid composition of phytoplankton. J. Phycol. 26: 278–288.
Volkman JK, Everitt DA, Allen DI (1986) Some analysis of lipid classes in marine organisms, sediments and seawater using thin-layer chromatography-flame ionization detection. J. Chromatogr. 356: 147–162.
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acids and lipid classes of ten species of microalgae used in mariculture. J. exp. mar. Biol. Ecol. 128: 219–240.
Webb KL, Chu FE (1983) Phytoplankton as a food source for bivalve larvae. In Pruder GD, Langdon CJ, Conklin DE (eds), Proc. of the 2nd int. conf. aquaculture nutrition, World Mariculture Society, Spec. Publ. No. 2, Louisiana State University, Louisiana, pp. 272–291.
Whyte JNC, Bourne N, Hodgson CA (1989) Influence of algal diets on biochemical composition and energy reserves inPatinopecten yessoensis (Jay) larvae. Aquaculture 78: 333–347.
Wikfors GH, Twarog JW, Ukeles R (1984). Influence of chemical composition of algal food sources on growth of juvenile oysters,Crassostrea virginica. Biol. Bull., 167: 251–263.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, M.R., Garland, C.D., Jeffrey, S.W. et al. The gross and amino acid compositions of batch and semi-continuous cultures ofIsochrysis sp. (clone T.ISO),Pavlova lutheri andNannochloropsis oculata . J Appl Phycol 5, 285–296 (1993). https://doi.org/10.1007/BF02186231
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02186231