Abstract
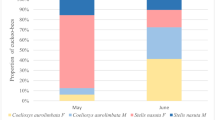
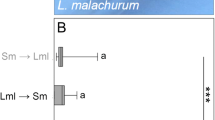
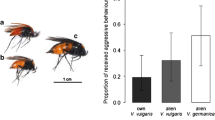
Nest invasion behavior was studied in six kleptoparasiticSphecodes species at four nesting sites of their respective social and solitary hosts.Sphecodes females preferred to enter unguarded nests. Nest intruding strategies observed in the differentSphecodes species did not depend on whether host species were solitary or social, as long as the nesting cycle of a social host was in the solitary stage (i.e., a single host female). Observation of intranidal behavior revealed thatSphecodes monilicornis females kill all host individuals within an usurped nest. They stay in the nest for several hours, laying eggs in adequately provisioned brood cells. Gas chromatography-mass spectrometry analyses of Dufour's gland secretions revealed species-specific compositions. Qualitative comparisons of whole patterns and quantitative comparisons considering the predominant hydrocarbons common to both host and parasite contradict the hypothesis of chemical mimetism, a mechanism supposed to permit parasite intrusion by qualitatively similar odor bouquets in host and parasite females.
Similar content being viewed by others
References
Alexander, B., and Rozen, J. G., Jr. (1987). Ovaries, ovarioles, and oocytes in parasitic bees (Hymenoptera: Apoidea).Pan-Pacif. Entomol. 63: 155–164.
Ayasse, M. (1991).Chemische Kommunikation bei der primitiv eusozialen Furchenbiene Lasioglossum malachurum (Halictidae): Ontogenese kastenspezifischer Duftstoffbouquets, Paarungsbiologie und Nesterkennung, Doctoral thesis, University of Tübingen, Germany.
Ayasse, M., Leys, R., Pamilo, P., and Tengö, J. (1990). Kinship in communally nestingAndrena (Hymenoptera: Andrenidae) bees is indicated by composition of Dufour's gland secretions.Biochem. Syst. Ecol. 18: 453–460.
Ayasse, M., Engels, W., Hefetz, A., Tengö, J., Lübke, G., and Francke, W. (1993). Ontogenetic patterns of volatiles identified in Dufour's gland extracts from queens and workers of the primitively eusocial halictine bee,Lasioglossum malachurum (Hymenoptera: Halictidae).Insect. Soc. 40: 1–18.
Batra, S. W. T. (1968). Behavior of some social and solitary halictine bees within their nests: A comparative study (Hymenoptera: Halictidae).J. Kans. Entomol. Soc. 41: 120–133.
Bell, W. J., Breed, M. D., Richards, K. W., and Michener, C. D. (1974). Social, stimulatory and motivational factors involved in intraspecific nest defense of a primitively eusocial halictine bee,J. Comp. Physiol. 93: 173–181.
Bohart, G. E. (1970).The Evolution of Parasitism Among Bees, Utah State University Press, Logan.
Davies, N. B., Bourke, A. F. G., and Brooke, M. D. L. (1989). Cuckoos and parasitic ants: Interspecific brood parasitism as an evolutionary arms race.Trends Ecol. Evol. 4: 274–278.
Duffield, R. M., Fernandes, A., Lamb, C., Wheeler, J. W., and Eickwort, G. C. (1981). Macrocyclic lactones and isopentenyl esters in the Dufour's gland secretion of halictine bees.J. Chem. Ecol. 7: 319–331.
Eickwort, G. C., and Abrams, J. (1980). Parasitism of sweat bees in the genusAgapostemon by cuckoo bees in the genusNomada (Hymenoptera: Halictidae, Anthophoridae).Pan-Pacif. Entomol. 56: 144–152.
Eickwort, G. C., and Eickwort, K. R. (1972). Aspects of the biology of Costa Rican Halictine bees. III.Sphecodes kathleenae, a social kleptoparasite ofDialictus umbripennis (Hymenoptera: Halictidae).J. Kans. Entomol. Soc. 45: 529–541.
Felsenstein, J. (1986).PHYLIP (Phylogeny Interference Package, Version 2.9).
Field, J. (1992). Intraspecific parasitism as an alternative reproductive tactic in nest-building wasps and bees.Biol. Rev. 67: 79–126.
Fisher, R. M. (1984). Evolution and host specificity: A study of the invasion success of a specialized bumble bee social parasite.Can. J. Zool. 62: 1641–1644.
Hefetz, A., Bergström, G., and Tengö, J. (1986). Species, individual and kin specific blends in Dufour's gland secretions of halictine bees—chemical evidence.J. Chem. Ecol. 12: 197–208.
Heide, A. v. D. (1992). Zur Bionomie vonLasioglossum (Evylaeus) fratellum (Pérez), einer Furchenbiene mit ungewöhnlich langlebigen Weibchen (Hymenoptera, Halictinae).Drosera '92(2): 89–206.
Johansson, I., Svensson, B. G., Tengö, J., and Bergström, G. (1982). Systematic relationship of halictine bees based on the pattern of macrocyclic lactones in the Dufour gland secretion.Insect Biochem. 12: 161–170.
Knerer, G. (1969). Stones, cement and guards in halictine nest architecture and defense.Entomol. News 80: 141–147.
Knerer, G. (1973). Periodizität und Strategie der Schmarotzer einer sozialen Schmalbiene,Evylaeus malachurus (K.) (Apoidea: Halictidae).Zool. Anz. 190: 41–63.
Knerer, G. (1980). Biologie und Sozialverhalten von Bienenarten der GattungHalictus Latreille (Hymenoptera: Halictidae).Zool. Jb. Syst. 107: 511–536.
Knerer, G., and Atwood, C. E. (1967). Parasitization of social halictine bees in southern Ontario.Proc. Entomol. Soc. Ontario 97: 103–110.
Knerer, G., and Plateaux-Quénu, C. (1967). Usurpation des nids etrangers et parasitisme facultatif chezHalictus scabiosae (Rossi).Insectes Soc. 14: 47–50.
Kronenberg, S., and Hefetz, A. (1984). Role of labial glands in nesting behaviour ofChalicodoma sicula (Hymenoptera; Megachilidae).Physiol. Entomol. 9: 175–179.
Legewie, H. (1925). Zum Problem des tierischen Parasitismus I. Teil: Die Lebensweise der SchmarotzerbieneSphecodes monilicornis K. (=subquadratus SM.) (Hym.: Apidae).Z. Morph. Ökol. Tiere 4: 430–464.
Michener, C. D. (1978). The parasitic groups of Halictidae (Hymenoptera, Apoidea).Univ. Kans. Sci. Bull. 51: 291–339.
Michener, C. D., and Brothers, D. J. (1971). A simplified observation nest for burrowing bees.J. Kans. Entomol. Soc. 44: 236–239.
Ordway, E. (1964).Sphecodes pimpinellae and other enemies ofAugochlorella.J. Kans. Entomol. Soc. 37: 139–152.
Packer, L. (1986). The biology of a subtropical population ofHalictus ligatus. 4. A cuckoo-like caste.J. N. Y. Entomol. Soc. 94: 458–466.
Prestwich, G. D., and Collins, M. S. (1981). Macrocyclic lactones as the defense substances of the termite genusArmitermes.Tetrahedron Lett. 22: 4587–4590.
Sick, M., Ayasse, M., Tengö, J., Lübke, G., and Francke, W. (1991). Spielen Duftstoffe eine Rolle bei der Fortpflanzung von Kuckucksibienen der GattungSphecodes (Hymenoptera: Halictidae)?Verh. Dtsch. Zool. Ges. 84: 393.
Tengö, J., and Baur, B. (1993). Fecundity in the kleptoparasitic beeNomada lathburiana (Anthophoridae): Number and size of oocytes in relation to body size and time of day.Entomol. Gen. 8: 19–24.
Tengö, J., and Bergström, G. (1976). Odor correspondence betweenMelitta females and males of their nest parasiteNomada flavopicta K. (Hymenoptera: Apoidea).J. Chem. Ecol. 2: 57–65.
Tengö, J., and Bergström, G. (1977). Cleptoparasitism and odor mimetism in bees: DoNomada males imitate the odor ofAndrena females?Science 196: 1117–1119.
Tengö, J., Sick, M., Ayasse, M., Engels, W., Svensson, B. G., Lübke, G., and Francke, W. (1992). Species specificity of Dufour's gland morphology and volatile secretions in kleptoparasiticSphecodes bees (Hymenoptera: Halictidae).Biochem. Syst. Ecol. 20: 351–362.
Torchio, P. F. (1989). Biology, immature development, and adaptive behavior ofStelis montana, a cleptoparasite ofOsmia (Hymenoptera: Megachilidae).Ann. Entomol. Soc. Am. 82: 616–632.
Torchio, P. F., and Burdick D. J. (1988). Comparative notes on the biology and development ofEpeolus compactus Cresson, a cleptoparasite ofColletes kincaidii Cockerell (Hymenoptera, Anthrophoridae, Colletidae).Ann. Entomol. Soc. Am. 81: 626–636.
Wcislo, W. T. (1987). The roles of seasonality, host synchronicity, and behaviour in the evolution and distributions of nest parasites in Hymenoptera (Insecta) with special reference to bees.Biol. Rev. 62: 515–543.
Wcislo, W. T. (1989). Behavioral environments and evolutionary change.Annu. Rev. Ecol. Syst. 20: 137–169.
Wheeler, W. M. (1919). The parasitic Aculeata, a study in evolution.Proc. Am. Philos. Soc. 58: 1–40.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sick, M., Ayasse, M., Tengö, J. et al. Host-parasite relationships in six species ofSphecodes bees and their halictid hosts: Nest intrusion, intranidal behavior, and Dufour's gland volatiles (Hymenoptera: Halictidae). J Insect Behav 7, 101–117 (1994). https://doi.org/10.1007/BF01989830
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01989830