Abstract
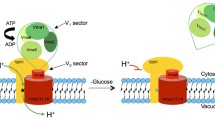
Vacuolar H+-adenosine triphosphatase (V-ATPase) is composed of distinct catalytic (V1) and membrane (V0) sectors containing several subunits. The biochemistry of the enzyme was mainly studied in organelles from mammalian cells such as chromaffin granules and clathrin-coated vesicles. Subsequently, mammalian cDNAs and yeast genes encoding subunits of V-ATPase were cloned and sequenced. The sequence information revealed the relation between V- and F-ATPases that evolved from a common ancestor. The isolation of yeast genes encoding subunits of V-ATPase opened an avenue for molecular biology studies of the enzyme. Because V-ATPase is present in every known eukaryotic cell and provides energy for vital transport systems, it was anticipated that disruption of genes encoding V-ATPase subunits would be lethal. Fortunately, yeast cells can survive the absence of V-ATPase by ‘drinking’ the acidic medium. So far only yeast cells have been shown to be viable without an active V-ATPase. In contrast to yeast, mammalian cells may have more than one gene encoding each of the subunits of the enzyme. Some of these genes encode tissue- and/or organelle-specific subunits. Expression of these specific cDNAs in yeast cells may reveal their unique functions in mammalian cells. Following the route from mammals to yeast and back may prove useful in the study of many other complicated processes.
Similar content being viewed by others
References
Harvey W. R. and Nelson N. (1992) V-ATPases, Cambridge, The Company of Biologists Limited
Mellman I., Fuchs R. and Helenius A. (1986) Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem.55: 663–700
Bowman B. J. and Bowman E. J. (1986) H+-ATPases from mitochondria, plasma membranes and vacuoles of fungal cells. J. Membr. Biol.94: 83–97
Nelson N. (1989) Structure, molecular genetics and evolution of vacuolar H+-ATPases. J. Bioenerg. Biomembr.21: 553–571
Nelson N. (1992) Evolution of organellar proton-ATPases. Biochim. Biophys. Acta1100: 109–124
Klionsky D. J., Herman P. K. and Emr S. D. (1990) The fungal vacuole: composition, function and biogenesis. Microbiol. Rev.54: 266–292
Cunningham K. W. and Fink G. R. (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases inSaccharomyces cerevisiae. Mol. Cell Biol.16: 2226–2237
Cunningham K. W. and Fink G. R. (1994) Calcineurin-dependent growth control inSaccharomyces cerevisiae mutants lackingPMC1. J. Cell Biol.124: 351–363
Cunningham K. W. and Fink G. R. (1994) Ca2+ transport inSaccharomyces cerevisiae. J. Exp. Biol.196: 157–166
Banta L. M., Robinsin J. S., Klionsky D. J. and Emr S. D. (1988) Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol.107: 1369–1383
Klionsky D. J., Nelson H. and Nelson N. (1992) Compartment acidification is required for efficient sorting of proteins to the vacuole inSaccharomyces cereviseae. J. Biol. Chem.267: 3416–3422
Yaver D. S., Nelson H., Nelson N. and Klionsky D. J. (1993) Vacuolar ATPase mutants accumulate precursor proteins in a pre-vacuolar compartment. J. Biol. Chem.268: 10564–10572
Morano K. A. and Klionsky D. J. (1994) Differential effects of compartment deacidification on the targeting of membrane and soluble proteins to the vacuole in yeast. J. Cell Sci.107: 2813–2824
Gluck S. (1992) V-ATPases of the plasma membrane. J. Exp. Biol.172: 29–37
Gluck S. and Nelson R. (1992) The role of the V-ATPase in renal epithelial H+ transport. J. Exp. Biol.172: 205–218
Gluck S. L. (1992) The structure and biochemistry of the vacuolar H+ ATPase in proximal and distal urinary acidification. J. Bioenerg. Biomembr.24: 351–359
Vaes G. (1988) Cellular biology and biochemical mechanism of bone resorption. Clin. Orthop.231: 239–271
Blair H. C., Teitelbaum S. L., Ghiselli R. and Gluck S. (1989) Osteoclastic bone resorption by a polarized vacuolar proton pump. Science245: 855–857.
Arnett T. R. and Dempster D.W. (1986) The effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology119: 119–124
Chatterjee D., Chakraborty M., Leit M., Neff L., Samsa-Kellokumpu S., Fuchs R. et al. (1992) Sensitivity to vanadate and isoforms of subunits A and B distinguish the osteoclast proton-pump from other vacuolar H+-ATPases. Proc. Natl. Acad. Sci. USA89: 6257–6291
Chatterjee D., Neff, L., Chakraborty M., Fabricant C. and Baron R. (1993) Sensitivity to nitrate and other oxyanions further distinguishes the vanadate-sensitive osteoclast proton pump from other vacuolar H+-ATPases. Biochemistry32: 2808–2812
David P. and Baron R. (1994) The catalytic cycle of the vacuolar H+-ATPase: comparison of proton transport in kidney- and osteoclast-derived vesicles. J. Biol. Chem.269: 30158–30163
Vaananen H. K., Karhukorpi E. K., Sundquist K., Wallmark B., Roininen I., Hentunen T. et al. (1990) Evidence for the presence of a proton pump of the vacuolar H+-ATPase type in the rulffled border of osteoclasts. J. Cell Biol.111: 1305–1311
Cidon, N. and Nelson N. (1986) Purification ofN-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J. Biol. Chem.261: 9222–9227
Percy J. M. and Apps D. K. (1986) Proton-translocating adenosine triphosphatase of chromaffin-granule membranes, the active site is in the largest (70 kDa) subunit. Biochem. J.239: 77–81
Moriyama Y. and Nelson N. (1987) The purified ATPase from chromaffin granule membranes is an anion-dependent proton pump. J. Biol. Chem.262: 9175–9180
Gluck S. and Caldwell J. (1987) Immunoaffinity purification and characterization of vacuolar H+-ATPase from bovine kidney. J. Biol. Chem.262: 15780–15789
Arai H., Berne M., Terres G., Terres H., Puopolo K. and Forgac M. (1987) Subunit composition and ATP site labeling of the coated vesicle proton- translocating adenosinetriphosphatase. Biochemistry26: 6632–6638
Xie X.-S. and Stone D. K. (1986) Isolation and reconstitution of the clathrin-coated vesicles proton translocating ATPase complex. J. Biol. Chem.261: 2492–2495
Xie X.-S. and Stone D. K. (1988) Partial resolution and reconstitution of the subunits of the clathrin-coated vesicle proton ATPase responsible for Ca2+-activated ATP hydrolysis. J. Biol. Chem.263: 9859–9867
Boyer P. D. (1993). The binding change mechanism for ATP synthase: some probabilities and possibilities. Biochim. Biophys. Acta1140: 215–250
Kane P. M., Yamashiro C. T. and Stevens T. H. (1989) Biochemical characterization of the yeast vacuolar H+-ATPase. J. Biol. Chem.264: 19236–19244
Umemoto N., Ohya Y. and Anraku Y. (1991)VMA11, a novel gene that encodes a putative proteolipid, is indispensable for expression of yeast vacuolar membrane H+-ATPase activity. J. Biol. Chem.266: 24526–24532
Hirata R., Umemoto N., Ho M. N., Ohya Y., Stevens T. H. and Anraku, Y. (1993)VMA12 is essential for assembly of the vacuolar H+-ATPase subunits onto the vacuolar membrane inSaccharomyces cerevisiae. J. Biol. Chem.268: 961–967
Ho M. N., Hirata R., Umemoto N., Ohya Y., Takatsuki A., Stevens T. H. et al. (1993)VMA13 encodes a 54-kDa vacuolar H+-ATPase subunit required for activity but not assembly of the enzyme complex inSaccharomyces cerevisiae. J. Biol. Chem.268: 18286–18292
Hill K. J. and Stevens T. H. (1994) Vma 21p is a yeast membrane protein retained in the endoplasmic reticulum by a Di-lysine motif and is required for the assembly of the vacuolar H+-ATPase complex. Molecular Biology of the Cell5: 1039–1050
Hirata R., Ohsumi Y., Nakano A., Kawasaki H., Suzuki K. and Anraku Y. (1990) Molecular structure of a gene,VMA1, encoding the catalytic subunit of H+-translocating adenosine triphosphatase from vacuolar membranes ofSaccharomyces cerevisiae. J. Biol. Chem.265: 6726–6733
Nelson H., Mandiyan S. and Nelson N. (1989) A conserved gene encoding the 57 kDa subunit of the yeast vacuolar H+-ATPase. J. Biol. Chem.264: 1775–1778
Puopolo K., Kumamoto C., Adachi I., Magner R. and Forgac M. (1992) Differential expression of the “B” subunit of the vacuolar H+-ATPase in bovine tissues. J. Biol. Chem.267: 3696–3706
Nelson H. and Nelson N. (1989) The progenitor of ATP synthases was closely related to the current vacuolar H+-ATPase. FEBS Lett.247: 147–153
Umemoto N., Yoshihisa T., Hirata R. and Anraku Y. (1990) Roles of theVMA3 gene product, subunitc of the vacuolar membrane H+-ATPase on vacuolar acidification and protein transport. J. Biol. Chem.265: 18447–18453
Foury F. (1990) The 31-kDa polypeptide is an essential subunit of the vacuolar ATPase inSaccharomyces cerevisiae. J. Biol. Chem.265: 18554–18560
Beltrán C., Kopecky J., Pan Y.-C. E., Nelson H. and Nelson N. (1992) Cloning and mutational analysis of the gene encoding subunit C of yeast V-ATPase. J. Biol. Chem.267: 774–779
Bauerle C., Ho M. N., Lindorfer M. A. and Stevens T. H. (1993) TheSaccharomyces cerevisiae VMA6 gene encodes the 36-kDa subunit of the vacuolar H+-ATPase membrane sector. J. Biol. Chem.268: 12749–12757
Nelson H., Mandiyan S. and Nelson N. (1994) TheSaccharomyces cerevisiae VMA7 gene encodes a 14-kDa subunit of the vacuolar H+-ATPase catalytic sector. J. Biol. Chem.269: 24150–24155
Graham L. A., Hill K. J. and Stevens T. H. (1994)VMA7 encodes a novel 14-kDa subunit of theSaccharomyces cerevisiae vacuolar H+-ATPase complex. J. Biol. Chem.269: 25974–25977
Nelson H., Mandiyan S. and Nelson N. (1995) A bovine cDNA and a yeast gene-VMA8 encoding subunit D of the vacuolar H+-ATPase. Proc. Natl. Acad. Sci. USA92: 497–501
Graham L. A., Hill K. J. and Stivens T. H. (1995)VMA8 encodes a 32-kDa V1 subunit of theSaccharomyce cerevisiae vacuolar H+-ATPase required for function and assembly of the enzyme complex. J. Biol. Chem.270: 15037–15044
Supekova L., Supek F. and Nelson N. (1995) TheSaccharomyces cerevisiae VMA10 is an intron-containing gene encoding a novel 13-kDa subunit of vacuolar H+-ATPase. J. Biol. Chem.270: 13726–13732
Manolson M. F., Proteau D., Preston R. A., Stenbit A., Roberts T., Hoyt M. et al. (1992) TheVPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J. Biol. Chem.267: 14294–14303
Manolson M. F., Wu B., Proteau D., Taillon B. E., Roberts B. T., Hoyt M. A. et al. (1994)STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J. Biol. Chem.269: 14064–14074
Moriyama Y. and Nelson N. (1989) Cold inactivation of vacuolar H+-ATPases. J. Biol. Chem.264: 3577–3582
Zhang J., Myers M. and Forgac M. (1992) Characterization of the V0 domain of the coated vesicle (H+)-ATPase. J. Biol. Chem.267: 9773–9778
Abrahams J. P., Leslie A. G. W., Lutter R. and Walker, J. E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature370: 621–628.
Arai H., Terres G., Pink S. and Forgac M. (1988) Topography and subunit stoichiometry of the coated vesicle proton pump. J. Biol. Chem.263: 8796–8802
Moriyama Y. and Nelson N. (1989) Lysosomal H+-translocating ATPase has a similar subunit structure to chromaffin granules H+-ATPase complex. Biochim. Biophys. Acta980: 241–247
Moriyama Y. and Nelson N. (1989) H+-translocating ATPase in Golgi apparatus: characterization as vacuolar H+-ATPase and its subunit structures. J. Biol. Chem.264: 18445–18450
Peng S.-B., Stone D. K. and Xie X.-S. (1993) Reconstitution of recombinant 40-kDa subunit of the clathrin-coated vesicle H+-ATPase. J. Biol. Chem.268: 23519–23523
Peng, S.-B., Zhang Y., Crider B. P., White A. E., Fried V. A., Stone D. K. and Xie X.-S. (1994) Reconstitution of the recombinant 70-kDa subunit of the clathrin-coated vesicle H+-ATPase. J. Biol. Chem.269: 27778–27782
Peng S.-B., Zhang, Y., Tsai S. J., Xie X.-S. and Stone D. K. (1994) Reconstitution of recombinant 33-kDa subunit of the clathrin-coated vesicle H+-ATPase. J. Biol. Chem.269: 11356–11360
Puopolo K. and Forgac M. (1990) Functional reassembly of the coated vesicle proton pump. J. Biol. Chem.265: 14836–14841
Forgac M. (1992) Structure and properties of the coated vesicle (H+)-ATPase. J. Bioenerg. Biomembr.24: 341–350
Nelson H. and Nelson N. (1990) Disruption of genes encoding subunits of yeast vacuolar H+-ATPase causes conditional lethality. Proc. Natl. Acad. Sci. USA87: 3503–3507
Noumi T., Beltrán C., Nelson H. and Nelson N. (1991) Mutational analysis of yeast vacuolar H+-ATPase. Proc. Natl. Acad. Sci. USA88: 1938–1942
Kane P. M., Kuehn M. C., Howald-Stevenson I. and Stevens T. H. (1992) Assembly and targeting of peripheral and integral membrane subunits of the yeast vacuolar H+-ATPase. J. Biol. Chem.267: 447–454
Supek F., Supekova L. and Nelson N. (1994) Features of vacuolar H+-ATPase revealed by yeast suppressor mutants. J. Biol. Chem.269: 26479–26485
Bowman E. J., Tenney K. and Bowman B. J. (1988) Isolation of genes encoding theNeurospora vacuolar ATPase. J. Biol. Chem.263: 13994–14001
Zimniak L., Dittrich P., Gogarten J. P., Kibak H. and Taiz L. (1988) The cDNA sequence of the 69 kDa subunit of the carrot vacuolar H+-ATPase. J. Biol. Chem.263: 9102–9112
Moriyama Y. and Nelson N. (1987) Nucleotide binding sites and chemical modification of the chromaffin granule proton ATPase. J. Biol. Chem.262: 14723–14729
Saraste M., Sibbald P. R. and Wittinghofer A. (1990) The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci.15: 430–434
Feng Y. and Forgac M. (1992) Cysteine 254 of the 73-kDa A subunit is responsible for inhibition of the coated vesicle (H+)-ATPase upon modification by sulfhydryl reagents. J. Biol. Chem.267: 5817–5822
Manolson M. F., Rea P. A. and Poole R. J. (1985) Identification of 3-O-(4-benzoyl)benzoyladenosine 5′-triphosphate-andN,N′-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J. Biol. Chem.260: 12273–12279
Zhang J., Vasilyeva E., Feng Y. and Forgac M. (1995) Inhibition and labeling of the coated vesicle V-ATPase by 2-azido-[32P]ATP. J. Biol. Chem.270: 15494–15500
Bowman B. J., Allen R., Wechser M. A. and Bowman E. J. (1988) Isolation of genes encoding theNeurospora vacuolar ATPase. J. Biol. Chem.263: 14002–14007
Senior A. E. (1990) The proton-translocating ATPase ofEscherichia coli. Annu. Rev. Biophys. Biophys. Chem.19: 7–41
Walker J. E., Lutter R., Dupuis A. and Runswick M. J. (1991) Identification of the subunits of F1F0-ATPase from bovine heart mitochondria. Biochemistry30: 5369–5378
Fillingame R. H. (1992) H+ transport and coupling by the F0 sector of the ATP synthase: insights into the molecular mechanism of function. J. Bioenerg. Biomembr.24: 485–491
Girvin M. E. and Fillingame R. H. (1993) Helical structure and folding of subunitc of F1F0 ATP synthase: 1H NMR resonance assignments and NOE analysis. Biochemistry32: 12167–12177
Girvin M. E. and Fillingame R. H. (1994) Hairpin folding of subunitc of F1F0 ATP synthase:1H distance measurements to nitroxide-derivatized aspartyl-61. Biochemistry33: 665–674
Mandel M., Moriyama Y., Hulmes J. D., Pan Y.-C. E., Nelson H. and Nelson N. (1988) Cloning of cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc. Natl. Acad. Sci. USA85: 5521–5524
Arai H., Berne M. and Forgac M. (1987) Inhibition of the coated vesicle proton pump and labeling of a 17,000 dalton polypeptide by DCCD. J. Biol. Chem.262: 11006–11011
Sze H., Ward J. M. and Lai S. (1992) Vacuolar H+-translocating ATPases from plants: structure, function, and isoforms. J. Bioenerg. Biomembr.24: 371–381
Supekova L., Sbia M, Supek F., Ma Y.-M. and Nelson N. (1996) A novel subunit of vacuolar H+-ATPase related to theb subunit of F-ATPases. J. Exp. Biol.119: 1147–1156
Dunn S. D. (1992) The polar domain of theb subunit ofEscherichia coli F1F0-ATPase forms an, elongated dimer that interacts with the F1 sector. J. Biol. Chem.267: 7630–7636
Vik S. B. and Dao N. N. (1992) Prediction of transmembrane topology of F0 proteins fromE. coli F1F0 ATP synthase using variational and hydrophobic moment analysis. Biochim. Biophys. Acta1140: 199–207
Wang S.-Y., Moriyama Y., Mandel M., Hulmes J. D., Pan Y.-C. E., Danho W. et al. (1989) Cloning of cDNA encoding a 32-kDa protein: an accessory polypeptide of the H+-ATPase from chromaffin granules. J. Biol. Chem.263: 17638–17642
Munn A. L. and Riezman H. (1994) Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six newEND genes. J. Cell Biol.127: 373–386
Ohya Y., Umemoto N., Tanida I., Ohta A., Iida H. and Anraku Y. (1991) Calcium-sensitivecls mutants ofSaccharomyces cerevisiae showing a pet− phenotype are ascribable to defects of vacuolar membrane H+-ATPase activity. J. Biol. Chem.266: 13971–13977
Stevens T. H. (1992) The structure and function of the fungal V-ATPase. J. Exp. Biol.172: 47–55
Jones P. C., Harrison M. A., Kim Y.-I., Finbow M. E. and Findlay J. B. C. (1994) Structure and function of the proton-conducting sector of the vacuolar H+-ATPase. Biochem. Soc. Trans.22: 247–255
Liu Q., Kane P. M., Newman P. R. and Forgac M. (1996) Site-directed mutagenesis of the yeast V-ATPase B subunit (Vma2p). J. Biol. Chem.271: 2018–2022
Ho M. N., Hill K. J., Lindorfer M. A. and Stevens T. H. (1993) Isolation of vacuolar membrane H+-ATPase-deficient yeast mutants; the VMA5 and VMA4 genes are essential for assembly and activity of the vacuolar H+-ATPase. J. Biol. Chem.268: 221–227
Doherty R. D. and Kane P. M. (1993) Partial assembly of the yeast vacuolar H+-ATPase in mutants lacking one subunit of the enzyme. J. Biol. Chem.268: 16845–16851
Tomashek J. J., Sonnenburg J. L., Artimovich J. M. and Klionsky D. J. (1996) Resolution of subunit interactions and cytoplasmic sub-complexes of the yeast vacuolar proton-translocating ATPase. J. Biol, Chem.271: 10397–10404
Puopolo K., Sczekan M., Magner R. and Forgac M. (1992) The 40-kDa subunit enhances but is not required for activity of the coated vesicle proton pump. J. Biol. Chem.267: 5171–5176
Nelson H. and Nelson N. (1990) Disruption of genes encoding subunits of yeast vacuolar H+-ATPase causes conditional lethality. Proc. Natl. Acad. Sci. USA87: 3503–3507
Hill K. J. and Stevens T. H. (1994) Vma21p is a yeast membrane protein retained in the endoplasmic reticulum by a Di-lysine motif and is required for the assembly of the vacuolar H+-ATPase complex. Mol. Biol. Cell5: 1039–1050
Supek F., Supekova L., Mandiyan S., Pan Y.-C. E., Nelson H. and Nelson N. (1994) A novel accessory subunit for vacuolar H+-ATPase from chromaffin granules. J. Biol. Chem.269: 24102–24106
Pan Y. X., Gu H. H., Xu J. and Dean G. E. (1993)Saccharomyces cerevisiae expression of exogenous vacuolar ATPase subunit B. Biochim. Biophys. Acta1151: 175–185
Supek F., Supekova L., Beltrán C., Nelson H. and Nelson N. (1992) Structure, function, and mutational analysis of V-ATPases. Ann. NY Acad. Sci.671: 284–292
Schatz G. and Dobberstein B. (1996) Common principles of protein translocation across membranes. Science271: 1519–1526
Nelson N. and Schatz G. (1979) Energy dependent processing of cytoplasmically-made precursors to mitochondrial proteins. Proc. Natl. Acad. Sci. USA76: 4365–4370
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nelson, N., Klionsky, D.J. Vacuolar H+-ATPase: From mammals to yeast and back. Experientia 52, 1101–1110 (1996). https://doi.org/10.1007/BF01952108
Issue Date:
DOI: https://doi.org/10.1007/BF01952108