Summary
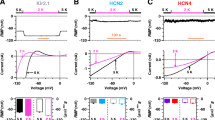
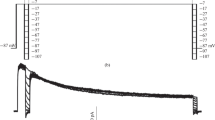
Inward currents carried by external Cs, Rb, NH4 and K through theI K1 channel were studied using a whole-cell voltage clamp technique. Cs, NH4, and Rb currents could be recorded negative to −40 mV following depolarizing prepulses (≥0 mV and 200–1000 msec in duration). The current activation displayed an instantaneous component followed by a monoexponential increase (τα) to a peak amplitude. Subsequent inactivation was fit by a single exponential, τiα. With hyperpolarization, τα and τiα decreasede-fold per 36 and 25 mV, respectively. In Ca-free external solutions (pipette [Mg]≈0.3mm), inactivation was absent, consistent with the hypothesis that inactivation represents time- and voltage-dependent block of Cs, NH4, and Rb currents by external Ca. The inactivation and degree of steady-state block was greatest when Cs was the charge carrier, followed by NH4, and then Rb. K currents, however, did not inactivate in the presence of Ca. Na and Li did not carry any significant current within the resolution of our recordings. Comparison ofpeak inward current ratios (I x/IK) as an index of permeability revealed a higher permeance of Cs (0.15), NH4 (0.30), and Rb (0.51) relative to K (1.0) than that obtained by comparing thesteady-state current ratios (Cs∶NH4∶Rb∶K≈0.01∶0.06∶0.21∶1.0). At any given potential, τα was smaller the more permeant the cation. In the absence of depolarizing prepulses, the amplitude of τα was reduced. Divalent-free solutions did not significantly affect activatio in the presence of 0.3mm pipette [Mg]. When pipette [Mg] was buffered to ≈50 μm, however, removal of external Ca and Mg lead to a four- to fivefold increase in Cs currents and loss of both time-dependent activation and inactivation (reversible upon repletion of external Ca).
These results suggest that (i) permeability ratios forI K1 should account for differences in the degree to which monovalent currents are blocked by extracellular Ca and (ii) extracellular or intracellular divalent cations contribute to the slow phase of activation which may represent either (a) the actual rate of Mg or Ca extrusion from the channel into the cell, a process which may be enhanced by repulsive interaction with the incoming permeant monovalent cation or (b) an intrinsic gating process that is strongly modulated by the permeant monovalent ion and divalent cations.
Similar content being viewed by others
References
Argibay, J.A., Dutey, P., Ildefonse, M., Ojeda, C., Rougier, O., Tourneur, Y. 1983. Block by Cs of K currentsI K1 and of carbachol induced current in frog atrium.Pfluegers Arch. 397:295–299
Armstrong, C.M. 1981. Sodium channels and gating currents.Physiol. Rev. 61:644–683
Armstrong, C.M., Lopez-Barneo, J. 1987. External calcium ions are required for potassium channel gating.Science 236:712–714
Armstrong, C.M., Swenson, R.P., Taylor, R. 1982. Block of squid axon K channels by internally and externally applied barium ions.J. Gen. Physiol. 80:663–682
Armstrong, C.M., Taylor, S.R. 1980. Interaction of barium ions with potassium channels in squid giant axons.Biophys. J. 30:473–488
Biermans, G., Vereecke, J., Carmeliet, E. 1987. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes.Pfluegers arch. 410:604–613
Cohen, I.S., DiFrancesco, D., Mulrine, N.K., Pennefather, P. 1989. Internal and external K gate the inward rectifier.Biophys. J. 55:197–202
Cota, G., Armstrong, C. 1988. Potassium channel “inactivation” induced by soft-glass pipettes.Biophys. J. 53:107–109
Frankenhaeuser, B., Hodgkin, A.L. 1957. The action of calcium on the electrical properties of squid axons.J. Physiol. 137:218–244
Fukushima, Y. 1982. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording.J. Physiol. 331:311–331
Gay, L.A., Stanfield, P.R. 1977. Cs causes a voltage dependent block of inward K currents in resting skeletal muscle fibers.Nature 267:169–170
Grissmer, S., Cahalan, M.D. 1989. Divalent ion trapping inside potassium channels of human T lymphoocytes.J. Gen. Physiol. 93:609–630
Hagiwara, S., Miyazaki, S., Krasne, S., Ciani, S. 1977. Anomalous permeabilities of the egg cell membrane of a starfish in K-T1 mixtures.J. Gen. Physiol. 70:269–281
Hagiwara, S., Miyazaki, S., Moody, W., Patlak, J. 1978. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg.J. Physiol. 279:167–185
Hagiwara, S., Miyazaki, S., Rosenthal, N.P. 1976. Potassium current and the effect of cesium ion on this current.J. Gen. Physiol. 67:621–638
Hagiwara, S., Takahashi, K. 1974. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell.J. Membrane Biol. 18:61–80
Hamill, O.P., Marty, A., Neher, E., Sakmann, B., Sigworth, F.J. 1981. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches.Pfluegers Arch. 391:85–100
Harvey, R., Ten Eick, R. 1988. Characterization of the inward-rectifying potassium current in cat ventricular myocytes.J. Gen. Physiol. 91:593–615
Hille, B. 1984. Ionic Channels of Excitable Membranes. p. 231. Sinauer, Sunderland, MA
Hille, B., Schwarz, W. 1978. Potassium channels as multi-ion single-file pores.J. Gen. Physiol. 72:409–442
Ishihara, K., Mitsuiye, T., Noma, A., Takano, M. 1989. The Mg2+ block and gating underlying inward rectification of the K+ current in guinea pig cardiac myocytes.J. Physiol. 419:297–320
Katz, B. 1949. Les constantes électriques de la membrane du muscle.Arch. Sci. Physiol. 2:285–299
Kurachi, Y. 1985. Voltage-dependent activation of the inward rectifier potassium channel in the ventricular cell membrane of guinea-pig heart.J. Physiol. 366:365–385
Latorre, R., Miller, C. 1983. Conduction and selectivity in potassium channels.J. Membrane Biol. 71:11–30
Martell, A.E., Smith, R.M. 1977. Critical Stability Constants. pp. 478, 651–652. Plenum, New York
Matsuda, H. 1988. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells.J. Physiol. 397:237–258
Matsuda, H., Saigusa, A., Irisawa, H. 1987. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg.Nature 325:156–159
Mitra, R., Morad, M. 1985. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates.Am. J. Physiol. 249:H1056-H1060
Mitra, R., Morad, M. 1987. Permeation and block of the inwardly rectifying K channel in isolated guinea pig ventricular myocytes by divalent and monovalent ions.J. Physiol. 382:128P
Mitra, R., Morad, M., Tourneur, Y. 1985. Time-dependent activation of the potassium inward rectifierI K1 in isolated guinea-pig cardiac cells.J. Physiol. 358:52P
Mitra, R., Vereecke, J., Carmeliet, E. 1990. Ca and Mg block an intrinsically high Na conductance through a cardiac K channel.Biophys. J. 57:111a
Neyton, J., Miller, C. 1988. Potassium blocks barium permeation through a calcium-activated potassium channel.J. Gen. Physiol. 92:549–567
Ohmori, H. 1978. Inactivation kinetics and steady state current noise in the anomalous rectifier of tunicate egg cell membranes.J. Physiol. 281:77–99
Robinson, R.A., Stokes, R.H. 1965. Electrolyte Solutions. p. 465. Butterworths, London
Sakmann, B., Trube, G. 1984. Voltage-dependent inactivation of inward-rectifying single channel currents in the guinea-pig heart cell membrane.J. Physiol. 347:659–683
Silver, M., DeCoursey, T.E. 1990. Intrinsic gating of the inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg.J. Gen. Physiol. 96:109–133
Standen, N.B., Stanfield, P.R. 1978. A potential and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions.J. Physiol. 280:169–191
Standen, N.B., Stanfield, P.R. 1979. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle.J. Physiol. 294:497–520
Tourneur, Y., Mitra, R., Morad, M., Rougier, O. 1987. Activation properties of the inward-rectifying potassium channel on mammalian heart cells.J. Membrane Biol. 97:127–135
Vandenberg, C. 1987. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions.Proc. Natl. Acad. Sci. USA 80:2560–2564
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mitra, R.L., Morad, M. Permeance of Cs+ and Rb+ through the inwardly rectifying K+ channel in guinea pig ventricular myocytes. J. Membrain Biol. 122, 33–42 (1991). https://doi.org/10.1007/BF01872737
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01872737