Summary
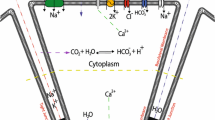
Models of epithelial salt secretion, involving secondary active transport of Cl− [9], locate the K+ conductance of the plasma membrane exclusively in the basolateral membrane, although there is considerable experimental evidence to show that many secretory epithelia do have a significant apical K+ conductance. We have used an equivalent circuit model to examine the effect of an apical K+ conductance on the composition and flow rate of the fluid secreted by an epithelium in which secretion is driven by the secondary active transport of Cl−. The parameters of the model were chosen to be similar to those measured in the dog tracheal mucosa when stimulated with adrenaline to secrete. We find that placing a K+ conductance in the apical membrane can actually enhance secretion provided that proportion of the total cell K+ conductance in the apical membrane is not greater than about 60%, the enabling effect on secretion being maximal when the proportion is around 10–20%. We also find that even when the entire cell K+ conductance is located in the apical membrane, the secreted fluid remains relatively Na+ rich. Analysis of the sensitivity of model behavior to the choice of values for the parameters shows that the effects of an apical K+ conductance are enhanced by increasing the ratio of the paracellular resistance to the transcellular resistance.
Similar content being viewed by others
References
Burgen, A.S.V., Emmelin, N.G. 1961. Physiology of the Salivary Glands. Arnold, London
Fuller, C.M., Eckhardt, L., Schulz, I. 1988. Ionic dependence of exocytosis from permeabilized acini of the rat pancreas.In: Exocrine Secretion. P.Y.D. Wong and J.A. Young, editors. pp. 65–67, Hong Kong University Press, Hong Kong
Greger, R., Schlatter, E. 1983. Properties of the basolatel membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney: A model for secondary active chloride transport.Pfluegers Arch. 396:325–334
Hodgkin, A.L., Katz, B. 1949. The effect of sodium ions on the electrical activity of the giant axon of the squid.J. Physiol. (London) 108:37–77
Lundberg, A. 1957. Secretory potentials in the sublingual gland of the cat.Acta Physiol. Scand. 40:21–34
Petersen, O.H., Maruyama, Y. 1984. Calcium-activated potassium channels and their role in secretion.Nature (London) 307:693–696
Poulsen, J.H., Laugesen, L.P., Nielsen, J.O.D. 1982. Evidence supporting that basolaterally located Na+-K+-ATPase and a co-transport system for sodium and chloride are key elements in secretion of primary saliva.In: Electrolyte and Water Transport Across Gastrointestinal Epithelia. R.M. Case et al., editors. pp. 157–159. Raven, New York
Shorofsky, S.R., Field, M., Fozzard, H.A. 1983. Electrophysiology of Cl secretion in canine trachea.J. Membrane Biol. 72:105–115
Silva, P., Stoff, J., Field, M., Fine, L., Forrest, J.N., Epstein, F.H. 1977. Mechanism of active chloride secretion by shark rectal gland: Role of Na−K-ATPase in chloride transport.Am. J. Physiol. 233:F298-F306
Smith, P.L., Frizzell, R.A., 1984. Chloride secretion by canine tracheal epithelium: IV. Basolateral membrane K permeability parallels secretion rate.J. Membrane Biol. 77:187–199
Suzuki, K., Petersen, O.H. 1985. The effect of Na+ and Cl− removal and of loop diuretics on acetylcholine-evoked membrane potential changes in mouse lacrimal acinar cells.Q. J. Exp. Physiol. 70:437–445
Welsh, M.J. 1983. Evidence for basolateral membrane potassium conductance in canine tracheal epithelium.Am. J. Physiol. 244:C377-C384
Welsh, M.J. 1983. Intracellular chloride activities in canine tracheal epithelium. Direct evidence for sodium-coupled intracellular chloride accumulation in a chloride-secreting epithelium.J. Clin. Invest. 71:1392–1401
Welsh, M.J. 1987. Electrolyte transport by airway epithelia.Physiol. Rev. 67:1143–1184
Welsh, M.J., Smith, P.L., Frizzell, R.A. 1983. Chloride secretion by canine tracheal epithelium: III. Membrane resistances and electromotive forces.J. Membrane Biol. 71:209–218
Young, J.A., Van Lennep, E.W. 1979. Transport in salivary and salt glands: I. Salivary glands.In: Membrane Transport in Biology. Vol. 4B, Transport Organs. G. Giebisch, D.C. Tosteson, and H.H. Ussing, editors. pp. 563–674. Springer-Verlag, Berlin
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cook, D.I., Young, J.A. Effect of K+ channels in the apical plasma membrane on epithelial secretion based on secondary active Cl− transport. J. Membrain Biol. 110, 139–146 (1989). https://doi.org/10.1007/BF01869469
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869469