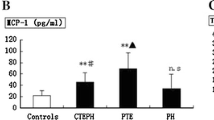
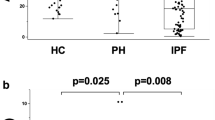
Summary
The aim of this study was to determine the value of von Willebrand factor (vWF), a well-characterized endothelial cell protein secretion, as a marker for prognosis in patients with primary pulmonary hypertension (PPH). Venous and arterial blood samples were obtained from 18 clinically diagnosed PPH patients and 12 case controls matched for age and sex. Plasma vWF antigen was determined by enzymelinked immunosorbent assay (ELISA). The patients' multimeric vWF pattern was analyzed by sodium dodecylsulfate (SDS)-agarose-acrylamide electrophoresis, Western blot, and densitometric analysis. vWF sialic acid content was determined by a lectin-based ELISA. The PPH patients showed a higher content of vWF antigen in venous (P = 0.0026) and arterial (P = 0.0094) blood samples than controls. The mean vWF sialic acid content of the PPH patients corresponded to 37.7% of the mean value for the control group. On the basis of the hemodynamic response to vasodilator trial, the PPH patients were grouped as responders or nonresponders. The latter group showed a significantly higher plasma vWF antigen antecubital vein/radial artery ratio, an increased number of unusually large vWF multimers, and a diminished content of vWF sialic acid in comparison with the first group. We believe that our results establish the nature of vWF alterations that are related to endothelial cell damage in patients with primary pulmonary hypertension and that this could be of value when establishing the prognosis in this group of patients.
Similar content being viewed by others
References
Wagner DD, Bonfanti R (1991) von Willebrand factor and the endothelium. Mayo Clin Proc 66:621–627
Mayer D, Gima JP (1993) von Willebrand factor: structure and function. Thromb Haemost 70:99–104
Handin IR, Wagner DD (1989) Molecular and cellular biology of von Willebrand factor. Prog Hemost Thromb 9:233–259
Sadler JE (1998) Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem 67:395–426
Samor B, Michalski JC, Mazurier C, Goudemand M, De Waard P, Vliegenthart JFG, Strtecker G, Montreuil J (1998) Primary structure of the major O-glycosidically linked carbohydrate unit of human von Willebrand factor. Glycoconj J 6:263–270
Mannucci PM (1998) von Willebrand factor. A marker of endothelial damage. Arterioscler Thromb Vasc Biol 18: 1359–1362
Tsang GM, Allen S, Pagano D, Wong C, Graham TR, Bonser RS (1998) von Willebrand factor and urinary albumin excretion are possible indicators of endothelial dysfunction in cardiopulmonary bypass. Eur J Cardiothorac Surg 13:385–391
Lip GYH, Blann AD (1995) von Willebrand and its relevance to cardiovascular disorders. Br Heart J 74:580–583
Mannucci PM, Lombardi R, Lattuada A, Perticucci E, Valsecchi R, Remuzzi G (1987) Suprarnormal von Willebrand factor multimers in scleroderma. Blood 73:1586–1591
López Fernández MF, López-Berges C, Martín R, Pardo A, Ramos FJ, Batle J (1987) Abnormal structure of von Willebrand factor in myeloproliferative syndrome is associated to either thrombotic or bleeding disorders. Thromb Haemost 58:753–757
Pedrinelli R, Giampietro O, Carmassi F, Melillo E, Dell'Omo G, Catapano G, Matteucci E, Talarico L, Morale M, De Negri F, Di Bello V (1994) Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet 344:14–18
Friedman R, Mears JG, Barst RJ (1997) Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation 96:2782–2784
Rich S, Brundage BH (1989) Pulmonary hypertension: a cellular basis for understanding the pathophysiology and treatment. J Am Coll Cardiol 14:545–550
Folta A, Joshua IG, Webb RC (1988) Endothelin-1-induced constriction in the coronary resistance vessels and abdominal aorta of the guinea pig. Heart Vessels 4:94–99
Loscalzo J (1992) Endothelial dysfunction in pulmonary hypertension. N Engl J Med 327:117–119
Rabinovitch M, Andrew M, Thom H, Trusler GA, Williams WG, Rowe RD, Olley PM (1987) Abnormal endothelial factor VIII associated with pulmonary hypertension and congenital heart defects. Circulation 76:1043–1052
Lopes AA, Maeda NY, Aiello VD, Ebaid M, Bydlowski SP (1993) Abnormal multimeric and oligomeric composition is associated with enhanced endothelial expression of von Willebrand factor in pulmonary hypertension. Chest 104:1455–1460
Lopes AA, Maeda NY (1998) Circulating von Willebrand factor antigen as a predictor of short-term prognosis in pulmonary hypertension. Chest 114:1276–1282
Welsh CH, Hassell KL, Badesch DB, Kressin DC, Marlar RA (1996) Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest 110: 710–717
Lopes AA, Maeda NY, Bydlowski SP (1998) Abnormalities in circulating von Willebrand factor and survival in pulmonary hypertension. Am J Med 105:21–26
Sandoval J, Bauerle O, Palomar A, Gómez A, Martínez-Guerra ML, Beltrán M, Guerrero L (1994) Survival in primary pulmonary hypertension. Circulation 89:1733–1744
Rich S (1993) Primary pulmonary hypertension. Curr Opin Cardiol 8:796–801
Grossmann R, Babin-Ebell J, Mishop M, Schwender S, Neukam K, Hickethier T, Elert O, Keller F (1991) Changes in coagulation and fibrinolytic parameters caused by extracorporeal circulation. Heart Vessels 6:102–106
Ruggeri ZM, Zimmerman TS (1981) The complex multimeric composition of factor VIII/von Willebrand factor. Blood 57:1140–1143
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA 76:4350–4354
Saulsbury FT (1997) Alterations in the O-linked glycosylation of IgAl in children with Henoch-Schonlein purpura. J Rheumatol 24:2246–2249
Varki A (1997) Sialic acids as ligands in recognition phenomena. FASEB J 11:248–255
Stewart DJ, Levy RD, Cernacek P, Langleben D (1991) Increased plasma endothelin 1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 114:464–469
Pearson JD (1993) Markers of endothelial cell perturbation and damage. Br J Rheumatol 32:651–652
Blann AD, Seigneur M (1997) Soluble markers of endothelial cell function. Clin Hemorrheol Microcirc 17:3–11
Blann AD, Taberner DA (1995) A reliable marker of endothelial cell dysfunction: does it exist? Br J Haematol 90:244–248
Rich S, Kaufman E, Levy PS (1992) The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 327:76–81
Behar S, Panosh A, Reicher Reiss H, Zion M, Schlesinger Z, Goldbourt U (1992) Prevalence and prognosis of chronic obstructive pulmonary disease among 5839 consecutive patients with acute myocardial infarction. SPRINT study group. Am J Med 93:637–641
Rossi A, Ziacchi V (1990) Hemodynamic effects of slowrelease nifedipine in severe congestive heart failure due to ischaemic heart disease. Cardiology 77:450–458
Badimon L, Badimon JJ, Chesebro JH, Fuster V (1993) von Willebrand factor and cardiovascular disease. Thromb Haemost 70:111–118
Badimon L, Badimon JJ, Penny W, Webster MW, Chesebro JH, Fuster V (1992) Endothelium and atherosclerosis. J Hypertens (suppl) 10:S43-S50
Geggel RL, Carvalho ACA, Hoyer LW, Reid LM (1987) von Willebrand factor abnormalities in primary pulmonary hypertension. Am Rev Respir Dis 135:294–299
Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colamino NM, Azocar J, Seder RH, Hong SL, Deykin D (1982) Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med 307:1432–1435
Visher UM, Wagner DD (1994) von Willebrand factor proteolytic processing and multimerization precede the formation of Weibel-Palade bodies. Blood 83:3536–3544
Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD (1986) Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear-stress induced platelet aggregation. J Clin Invest 78:1456–1458
Moake JL, Eisenstaedt RS (1994) Thrombotic thrombocytopenic purpura and the hemolytic uremic syndrome. In: Colman RW, Hirsch J, Marder VJ, Salzman EW (eds) Hemostasis and thrombosis: basic principles and clinical practice. Lippincott, Philadelphia, pp 1064–1075
Ribes JA, Francis CW, Wagner DD (1987) Fibrin induces release of von Willebrand factor from endothelial cells. J Clin Invest 79:117–123
Federici AB, Elder JH, De Marco L, Ruggeri ZM, Zimmerman TS (1984) Carbohydrate moiety of von Willebrand factor is not necessary for maintaining multimeric structure and ristocetin cofactor activity but protects from proteolytic degradation. J Clin Invest 74:2049–2055
Berkowitz SD, Federici AB (1988) Sialic acid prevents loss of large von Willebrand factor multimers by protecting againts amino-terminal proteolytic cleavage. Blood 72:1790–1796
Katz JA, Moake JL, McPherson PD, Weinstein MJ, Moise KJ, Carpenter RJ, Sala DJ (1989) Relationship between human development and disappearance of unusually large von Willebrand factor multimers from plasma. Blood 73:1851–1858
Alvarez G, Lascurain R, Pérez A, Degand P, Montaño LF, Martínez-Cairo S, Zenteno E (1999) Relevance of sialoglycoconjugates in murine thymocytes during maturation and selection in the thymus. Immunol Invest 28:9–18
Hassell KL (1998) Altered hemostasis in pulmonary hypertension. Blood Coagul Fibrinolysis 9:107–117
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Collados, M.T., Sandoval, J., López, S. et al. Characterization of von Willebrand factor in primary pulmonary hypertension. Heart Vessels 14, 246–252 (1999). https://doi.org/10.1007/BF01747854
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01747854