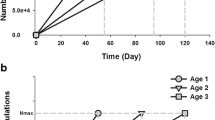
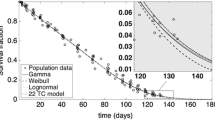
Summary
Direct in vivo labeling of erythrocytes with biotin is shown as a method for estimation of red cell survival as well as of enrichment of young or aged erythrocytes. Two succinimide esters (biotin-N-hydroxysuccinimide ester [BNHS], caproylamidobiotin-N-hydroxysuccinimide ester [C-BNHS] were used for biotin labeling of erythrocytes. With improved syntheses, pure BNHS (mp, 212°–214° C) and the spacered intermediate for C-BNHS, 6-(biotinylamide) hexanoate (mp, 225°–226° C) were obtained in an overall yield of 86%; the yield of C-BNHS (mp, 167°–169° C) was 68%. When three doses of 1 mg C-BNHS are injected intravenously into mice at 24-h intervals, all the red cells are biotin labeled. The rate of red cell production as well as the life span of red cells can be measured without any effect on erythropoiesis or damage by red cells in vitro. The survival curve seems to be linear, with 2.5%–3.3% disappearance of biotin-labeled red cells daily. In mice, in vivo biotin labeling avoids damaging red cells by in vitro procedures and does not influence the steady state of erythropoiesis by hypertransfusion. Therefore, in vivo biotin labeling is a very useful method for determining red cell survival time in small animals.
Similar content being viewed by others
References
Ashby W (1919) The determination of the length of the life of transfused blood corpuscles in man. J Exp Med 29: 267–281
Bross KJ (1980) Nachweis von Zellmembranantigenen mit einer Immunoperoxidase-Objektträgermethode. Habilitationsschrift, Universität Freiburg/Brsg
Burstone MS (1959) New histochemical techniques for the demonstration of tissue oxidase (cytochrome oxidase). J Histochem Cytochem 7: 112–122
Cavill I, Trevett D, Fisher J, Hoy T (1988) The measurement of the total volume of red cells in man: a non-radioactive approach using biotin. Br J Haematol 70: 491–493
Costello SM, Felix RT, Giese RW (1979) Enhancement of immune cellular agglutination by use of the avidin-biotin system. Clin Chem 25: 1572–1580
Dale GL, Norenberg SL (1990) Density fractionation of erythrocytes by Percoll/hypaque results in only a slight enrichment for aged cells. Biochim Biophys Acta 1036: 183–187
Gais P, Rodenacker K (1987) Hard- and software requirements of a multipurpose image analysis system. In: Burger G, Ploem JS, Goerttler K (eds) Clinical cytometry and histometry. Academic, London, pp 36–38
International Committee for Standardization in Hematology (1982) Recommended method for radioisotype red-cell survival studies. Br J Haematol 45: 659–666
Jasiewicz MJ, Schoenberg DR, Mueller GC (1976) Selective retrieval of biotin-labeled cells using immobilized avidin. Exp Cell Res 100: 213–217
Kranz B, Thierfelder S (1986) Improved detection of terminal transferase (TdT): the use of detergents on glutaraldehyde fixed non-dehydrated cells prevents denaturation and diffusion artifacts. Leukemia Res 10: 1041–1049
Kranz B, Thiel E, Thierfelder S (1989) Immunocytochemical identification of meningeal leukemia and lymphoma: Poly-L-lysine-coated slides permit multimarker analysis even with minute cerebrospinal fluid cell specimens. Blood 73: 1942–1950
Krzymowski T, Krzymowska H (1982) Studies on the erythropoiesis-inhibiting factor in the plasma of animals with transfusion polycythemia. Blood 19: 38–44
Lindemann R (1971) Erythropoiesis-inhibiting factor (EIF). I. Fractionation and demonstration of urinary EIF. Br J Haematol 21: 623–631
Magnani M, Rosse L, Stocchi V, Cucchiarini L, Piacentini G, Fornaini G (1988) Effect of age on some properties of mice erythrocytes. Mech Ageing Dev 42: 37–47
Mollison PL (1983) Blood transfusion in clinical medicine. Blackwell Scientific, Oxford
Putten LM Van (1958) The life span of red cells in the rat and the mouse as determined by labeling with DFP32 in vivo. Blood 13: 789–794
Slezak SE, Horan PK (1989) Fluorescent in vivo tracking of hematopoietic cells. I. Technical considerations. Blood 74: 2172–2177
Sluiter W, Hulsing-Hesselink E, Elzenga-Claasen I, Van Furth R (1985) Method to select mice in the steady state for biological studies. J Immunol Methods 760: 135–143
Suzuki T, Dale GL (1987) Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood 70: 791–795
Whitcomb WH, Moore MZ (1965) The inhibitory effect of plasma from hypertransfused animals on erythrocyte iron incorporation in mice. J Lab Clin Med 66: 641–651
Author information
Authors and Affiliations
Additional information
Supported by DFG (Ho 779/4-1)
Rights and permissions
About this article
Cite this article
Hoffmann-Fezer, G., Maschke, H., Zeitler, H.J. et al. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann Hematol 63, 214–217 (1991). https://doi.org/10.1007/BF01703446
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01703446