Summary
Following complete transection of the spinal cord at T 9, 12 cats were separated into two groups:
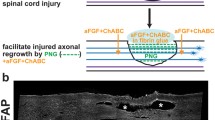
Group 1 received a collagen matrix (CM)* treated with a neuroactive agent or with saline to bridge the spinal cord stumps and an omental transposition which was placed on the dorsal surface of the matrix;
Group 2 received spinal cord transection only.
Two cats received no spinal cord transection.
After 90 days, all animals were killed and their brains and spinal cords were removed for immunohistochemical examination. Two weeks prior to sacrifice, spinal cord blood flows (SCBF) were measured and the retrograde axonal tracer Fluoro-Gold was injected below the transection site.
Results show that omental transposition to the CM bridge in Group 1 animals increased SCBF an average 59% (assessed by clamping the omental blood supply to the cord).
Examination of the brain 90 days after cord transection revealed Fluoro-Gold accumulation in the cytoplasm and processes of neurons located in the brainstem, midbrain, and diencephalic region which are known to contribute pathways to the spinal cord.
Immunohistochemical staining with antibodies against the catecholamine synthesizing enzymes tyrosine hydroxylase and dopamine-B-hydroxylase, indicated that only Group 1 treated cats developed dense bundles of dopaminergic and noradrenergic fibers within the CM bridge and distal spinal cord tissue. These fibers were seen to extend 90 mm below the transection site. In addition, the synaptogenic marker synaptophysin (SYN) was observed in association with dopaminergic and noradrenergic fibers distal to the collagen matrix bridge, an indication that synaptic remodelling (regeneration) by previously denervated supraspinal axons may have occured. Immunostaining for glial fibrillary acidic protein (GFAP) showed little to none reactive astrocytosis near the transection site of cats treated with the CM and omentum transposition (Group 1). No catecholaminergic fibers or SYN expression below the transection site were observed in Group 2 treated cats. Group 2 treated cats also showed dense immunostaining of GFAP near the transection site indicating significant astrocytic proliferation.
These findings indicate that following complete spinal cord transection in cats and reconstruction with a treated collagen matrix and omental transposition, disconnected supraspinal fibers have the ability to regenerate for long anatomic distances and seemingly engage in synaptic remodelling with distal target tissue.
Similar content being viewed by others
References
Breasted JH (1930) The Edwin Smith surgical papyrus, 2 volumes. The University of Chicago Press
Kiernan JA (1979) Hypotheses concerned with axonal regeneration in the mammalian nervous system. Biol Rev 54: 155–197
Kiernan JA (1978) An explanation of axonal regeneration in peripheral nerves and its failure in the central nervous system. Med Hypoth 4: 15–26
Berry M (1979) Regeneration in the central nervous system. Rec Adv Neuropath 67: 111–132
de la Torre JC (1984) Spinal cord injury models. Progr Neurobiol 22: 289–344
Ramón y Cajal S (1984) The Neuron and the gial cell. de la Torre JC, Gibson WC (eds/transl). Charles C Thomas, Springfield, Illinois
Ramón y Cajal S (1959) Degeneration and regeneration of the nervous system. May R (ed/transl). Hafner Publishing, New York, pp 749–750
de la Torre JC (1986) Experimental problems in spinal cord neural reconstruction. In: Gilad G, Gorio A, Kreutzberg G (eds) Process of recovery from neural trauma. Springer, New York, pp 317–325
de la Torre JC, Goldsmith HS (1990) Collagen-omental graft in experimental spinal cord transection. Acta Neurochir (Wien) 102: 152–163
de la Torre JC, Goldsmith HS (1988) Increased blood flow enhances axon regeneration after spinal transection. Neurosci Lett 94: 269–273
de la Torre JC, Hill PK, Gonzalez M, Parker JC (1983) Transected spinal cord axons regenerate into a collagen bioimplant. In: Haber B, Perez-Polo J, Hashim G (eds) Nervous system regeneration. Alan Liss, pp 515–520
Goldsmith HS, Duckett S, Chen W (1975) Spinal cord vascularization by intact omentum. Am J Surg 129: 262–265
Ingvar DH, Lassen NA (1962) Regional blood flow of the cerebral cortex determined by krypton 85. Acta Physiol Scand 54: 325–338
Sternberger LA (1986) Immunocytochemistry, 3rd ed. Wiley, New York
Hosoya Y (1980) The distribution of spinal projection neurons in the hypothalamus of the rat, studied with HRP method. Exp Br Res 40: 79–87
Goldsmith HS, Steward E, Chen W, Duckett S (1983) Application of the intact omentum on normal and injured spinal c. In: Kao CC, Bunge RP (eds) Spinal cord reconstruction. Raven Press, New York, pp 235–243
Zivin JA, Reid J, Saavedra J, Kopin I (1975) Quantitative localization of biogenic amines in cat spinal cord. Brain Res 99: 293–301
Fleetwood-Walker SM, Foote JH (1981) Contribution of noradrenaline, dopamine and adrenaline-containing axons to their innervation of differences regions of the spinal cord of the cat. Brain Res 2906: 95–106
Goldsmith HS, McIntosh T, Vezine R, Colton T (1987) Vasoactive neurochemicals identified in omentum: a preliminary report. Br J Neurosurg 1: 359–364
Siek G, Marquis J, Goldsmith HS (1987) Experimental studies of omentum-derived neurotrophic factors. In: Goldsmith HS (ed) The Omentum; Research and Clinical Applications. Springer, New York, pp 83–95
Jahne R, Schiebler W, Ouimet C, Greengard P (1985) A 3,000-dalton membrane protein (p 38) present in synaptic vesicles. Proc Natl Acad Sci 82: 4137–4141
Leclerc N, Beesley P, Brown I, Colonnier M, Gurd J, Paladina T, Hawkes R (1989) Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol 280: 197–212
Miller DC, Koslow M, Budzilovich G, Burstein D (1990) Synaptophysin: A sensitive and specific marker for ganglion cells in central nervous system neoplasm. Hum Path 21: 270–276
Hagihara S, Senba E, Yoshida S, Tohyama M, Yoshiya I (1990) Fine structure of noradrenergic terminals and their synapses in the rat spinal dorsal horn: An immunohistochemical study. Brain Res 526: 73–80
Yoshida M, Tanaka M (1988) Existence of new dopaminergic terminal plexus in the rat spinal cord: assessment by immunohistochemistry using anti-dopamine serum. Neurosci Lett 94: 5–9
Chiba T, Masuko S (1987) Synaptic structure of the monoamine and peptide nerve terminals in the intermediolateral nucleus of the guinea pig thoracic spinal cord. J Comp Neurol 262: 242–255
Ross RA, Reis DJ (1974) Effects of lesions of locus coeruleus on regional distribution of dopamine-β-hydroxylase activity in rat brain. Brain Res 73: 161–166
Westlund KN, Bowker R, Zeigler M, Coulter J (1983) Noradrenergic projections to the spinal cord. Brain Res 263: 15–31
Commissiong JW, Gentleman S, Neff N (1979) Spinal cord dopaminergic neurons: evidence for an uncrossed nigrospinal pathway. Neuropharmacol 18: 565–568
Dahlstrom A, Fuxe K (1965) Evidence for the existence of monoamine neurons in the cebntral nervous system. II Experimentally induced changes in the intraneuronal amine levels of bulbospinal neuronal system. Acta Physiol Scand 64 [Suppl 247]: 7–36
Kimura H, McGeer PL, Peng J, McGeer AG (1981) The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol 200: 151–201
Petras JM, Gummings JF (1972) Autonomic neurons in the spinal cord of the rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathectomy with fiber degeneration following dorsal and rhizotomy. J Comp Neurol 146: 189–218
deQuidt ME, Emson PC (1986) Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system, II, Immunohistochemical analysis. Neurosci 18: 545–618
Liuzzi FJ, Lasek R (1987) Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 237: 642–645
Preddy R, Malhotra S (1989) Reactive astrocytes in lesioned rat spinal cord: effect of neural transplants. Brain Res Bull 22: 81–87
Freddy R, Malhotra S, Das G (1988) Enhanced expression of a protein antigen by reactive astrocytes in lacerated spinal cord. J Neurosci Res 19: 397–404
Buchanan J, Nomes H (1986) Transplants of embryonic brainstem containing the locus coeruleus into spinal cord enhance the hindlimb flexion reflex in adult rats. Brain Res 381: 225–236
Anderson CR, McLachlan E, Srb-Christie O (1989) Distribution of sympathetic preganglionic neurons and monoaminergic nerve terminals in the spinal cord of the rat. J Comp Neurol 283: 269–284
Mouchet P, Manier M, Dietl M, Feuerstein C, Berod A (1986) Immunohistochemical study of catecholaminergic cell bodies in rat spinal cord. Brain Res Bull 16: 341–353
Ambalavanar R, Morris R (1989) Fluoro-Gold injected either subcutaneously or intravascularly results in extensive retrograde labelling of CNS neurons having axons terminating outside the blood-brain barrier. Brain Res 505: 171–175
Schmued L, Fallon J (1986) Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377: 147–154
Haymaker W, Adams R (1982) Histology and histopathology of the nervous system. Charles C Thomas 1: 34–35
Gelderd J (1990) Evolution of blood vessel and neuritic growth into a collagen matrix placed within a surgically created gap in rat spinal cord. Brain Res 511: 80–92
Cotman CW, Nieto-Sampedro M, Harris E (1981) Synapse replacement in the nervous system of adult vertebrates. Physiol Rev 61: 684–784
Brown MC, Holland R, Hopkins W (1981) Motor nerve sprouting. Ann Rev Neurosci 4: 17–81
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de la Torre, J.C., Goldsmith, H.S. Supraspinal fiber outgrowth and apparent synaptic remodelling across transected-reconstructed feline spinal cord. Acta neurochir 114, 118–127 (1992). https://doi.org/10.1007/BF01400599
Issue Date:
DOI: https://doi.org/10.1007/BF01400599