Summary
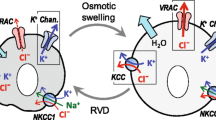
All cells including neurons and glial cells are able to keep their volume within a very limited range. The volume regulatory mechanism involves changes in the concentration of osmolytes of which taurine appears to be of particular importance in brain cells. Swelling in brain cells may occur as a result of depolarization or small fluctuations in osmolarity. In isolated brain cells these conditions will always lead to a release of taurine, the time course of which is superimposable on that of the volume regulatory decrease which follows the initial cell swelling. The mechanism responsible for taurine release associated with swelling has not been fully elucidated but a large body of evidence seems to exclude participation of the taurme high affinity carrier. Using a number of inhibitors of anion exchangers it has been demonstrated that both volume regulation and taurine release in brain cells are inhibited by these drugs, implicating an anion channel in the process. It has be controversial issue as to whether or not taurine release is Ca++ dependent. Recent evidence appears to suggest that the release process is not associated with Ca++ or Ca++ channels. It is, however, quite possible that the swelling process may involve the Ca++ calmodulin system or other second messengers. Taurine also contributes to volume regulation after shrinkage of brain cells, in this case by increasing its intracellular concentration. This change is accomplished byan upregulation of the Na+/taurine cotransporter, together with reduced passive fluxes and increased endogenous synthesis.
Similar content being viewed by others
References
Beetsch JW, Olson JE (1996) Hyperosmotic exposure alters total taurine quantity and cellular transport in rat astrocyte cultures. Biochim Biophys Acta 1290: 141–148
Bender AS, Neary JT, Norenberg MD (1992) Involvement of second messengers and protein phosphorylation in astrocyte swelling. Can J Physiol Pharmacol 70: S362-S366
Bender AS, Schousboe A, Norenberg MD (1995) Pharmacological characterization of glutamate-induced astrocyte swelling. Soc Neurosci 21: 1081
Benveniste H, Drejer J, Schousboe A, Diemer NH (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 43: 1369–1374
Bruhn T, Christensen T, Cobo M, Damgaard I, Diemer NH, Schousboe A (1996) Effects of phenylsuccinate on potassium stimulated taurine release in cultured neurons and astrocytes and in rat hippocampusin vivo. J Neurosci Res: 198–203
Cabantchik ZI, Greger R (1992) Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol 262: C803-C827
Chamberlain ME, Strange K (1989) Anisosmotic cell volume regulation: a comparative view. Am J Physiol 257: C159-C172
Dermietzel R, Hwang T, Buettener R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes FP, Fishman GI, Spray DC, Siemen D (1994) Cloning and in situ localization of a brain-derived porin that constitutes a large conductance anion channel in astrocytic plasma membranes. Proc Natl Acad Sci USA 86: 499–503
Goldstein L, Brill SR (1991) Volume-activated taurine efflux from skate erythrocytes: possible band 3 involvement. Am J Physiol 260: R1014–1020
Gonzălez E, Sănchez-Olea R, Pasantes-Morales H (1995) Inhibition by Cl channel blockers of the volume activated, diffusional mechanism of inositol transport in primary astrocytes in culture. Neurochem Res 20: 895–900
Hagberg H, Lehmann A, Sandberg M, Nyström B, Jacobsen I, Hamberger A (1985) Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab 5: 413–419
Hansen AJ (1985) Effects of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148
Hertz L, Schousboe A (1975) Ion and energy metabolism of the brain at the cellular level. Int Rev Neurobiol 18: 141–211
Hilgier W., Olson JE. (1994) Brain ion and amino acid contents during edema development in hepatic encephalopathy. J Neurochem 62: 197–204
Higgins CF (1995) P-glycoprotein and cell volume-activated chloride channels. J Bioenerg Biomembr 27: 63–70
Holopainen I, Oja SS, Marnela K-M, Kontro P (1986) Free amino acids in primary culture: changes during cell maturation. Int J Devl Neurosci 4: 493–496
Isaaks RE, Bender AS, Kim CY, Prieto NM, Norenberg MD (1994) Osmotic regulation of myo-inositol uptake in primary astrocyte cultures. Neurochem Res 19: 331–338
Jackson PS, Strange K (1993) Volume-sensitive anion channels mediate swellingactivated inositol and taurine efflux. Am J Physiol 265: C1489-C1500
Kay MMB, Hughes J, Azgón I, Lin F (1991) Brain membrane protein 3 performs the same functions as erythrocyte band 3. Proc Natl Acad Sci USA 88: 2778–2782
Kimelberg HK, Frangakis M (1986) Volume regulation in primary astrocyte cultures. Adv Biosci 67: 177–186
Kimelberg HK, Ransom BR (1986) Physiological and pathological aspects of astrocytic swelling. In: Fedoroff S, Vernadakis A (eds) Astrocytes, vol 3. Academic Press, New York, pp 129–166
Koyama Y, Ishibashi T, Tanaka K, Baba A (1994) L-Glutamate-stimulated taurine release from rat cerebral cultured astrocytes. J Neurosci Res 38: 75–80
Law RO (1989) The effects of pregnancy on the content of water, taurine and total amino nitrogen in rat cerebral cortex. J Neurochem 53: 300–302
Law RO (1994) Taurine efflux and the regulation of cell volume in incubated slices of rat cerebral cortex. Biochim Biophys Acta 1221: 21–28
Lehmann A (1989) Effects of microdialysis-perfusion with anisoosmotic media on extracellular amino acids in the rat hippocampus and skeletal muscle. J Neurochem 53: 525–535
Lehmann A (1990) Derangement in cerebral osmohomeostasis: a common denominator for stimulation of taurine and phsophoethanolamine release. In: Pasantes-Morales H, Martin D, Martìn del Rio W, Martìn del Rio R (eds) Taurine: functional biochemistry, physiology and cardiology. Wiley Liss, New York, pp 337–348
Levi G, Patrizio M (1992) Astrocyte heterogeneity: endogenous amino acid levels and release evoked by non-N-methyl-D-aspartate receptor agonists and by potassiuminduced swelling in type-1 and type-2 astrocytes. J Neurochem 58: 1943–1952
Liu MY, Colombini M (1992) Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim Biophys Acta 1098: 255–260
Macknight ADC (1988) Principles of cell volume regulation. Renal Physiol Biochem 3: 114–141
McManus ML, Churchwell KB (1994) Clinical significance of cellular osmoregulation. In: Strange K (ed) Cellular and molecular physiology of cell volume regulation. CRC Press, Boca Raton, pp 63–74
Moorman JR, Ackerman SJ, Kowdley GC, Griffin MP, Mounsey JP, Chen Z, Cala SE, O'Brian JJ, Szabo G, Jones LR (1995) Unitary anion currents through phospholemman channel molecules. Nature 377: 737–740
Nagelhus EA, Lehmann A, Ottersen OP (1993) Neuronal-glial exchange of taurine during hypo-osmotic stress: a combined immunocytochemical and biochemical analysis in rat cerebellar cortex. Neuroscience 54: 615–631
Nieminen ML, Tuomisto L, Solatunturi E, Eriksson L, Paasonen MK (1998) Taurine in the osmoregulation of the Brattelboro rat. Life Sci 42: 2137–2143
Oja SS, Kontro P (1983) Taurine. In: Lajtha A (ed) Taurine. Plenum Press, New York, pp 501–533
Ottersen OP (1988) Quantitative assessment of taurine-like immunoreactivity in different cell types and processes in rat cerebellum: an electronmicroscopic study based on a postembedding immunogold labelling procedure. Anat Embryol 178: 407–421
Pasantes-Morales H, Martìn del Rio M (1990) Taurine and mechanisms of cell volume regulation. In: Pasantes-Morales H, Martin DL, Shain W, Martìn del Rio R (eds) Taurine: functional neurochemistry, physiology, and cardiology. Wiley-Liss Inc., New York, pp 317–328
Pasantes-Morales H, Schousboe A (1988) Volume regulation in astrocytes: a role for taurine as osmoeffector. J Neurosci Res 20: 505–509
Pasantes-Morales H, Schousboe A (1989) Release of taurine from astrocytes during potassium-evoked swelling. Glia 2: 45–50
Pasantes-Morales H, Moran J, Schousboe A (1990) Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia 3: 427–432
Pasantes-Morales H, Maar T, Morăn J (1993) Cell volume regulation in cultured cerebellar granule neurons. J Neurosci Res 34: 219–224
Pasantes-Morales H, Murray RA, Lilja L, Morăn J (1994a) Regulatory volume decrease in cultured astrocytes I. Potassium- and chloride-activated permeability. Am J Physiol C165–C171
Pasantes-Morales H, Murray RA, Sănchez-Olea R, Morăn J (1994b) Regulatory volume decrease in cultured astrocytes: 11. Activated permeability to amino acids and polyalcohols. Am J Physiol 266: C172-C178
Pasantes-Morales H, Chacń E, Murray RA, Morăn J (1994c) Properties of osmolyte fluxes activated during regulatory volume decrease in cultured cerebellar granule neurons. J Neurosci Res 37: 720–727
Passarella S, Atlante A, Barile M, Quagliariello E (1987) Anion transport in rat brain mitochondria: Fumarate uptake via the dicarboxylate carrier. Neurochem Res 12: 255–264
Patel AJ, Hunt AH (1985) Concentration of free amino acids in primary cultures of neurones and astrocytes. J Neurochem 44: 1816–1821
Paulmichl M, Geschwentner M, Wöll E, Schmarda A, Ritter M, Kanin G, Ellemunter H, Waitz W, Deetjen P (1993) Insight into the structure-function relation of chloride channels. Cell Physiol Biochem 3: 374–387
Roy G (1995) Amino acid current through anion channels in cultured human glial cells. J Membr Biol 147: 35–44
Sănchez-Olea R, Morăn J, Schousboe A, Pasantes-Morales H (1991) Hyposmolarityactivated fluxes of taurine in astrocytes are mediated by diffusion. Neurosci Lett 130: 233–236
Sănchez-Olea R, Moran J, Pasantes-Morales H (1992) Changes in taurine transport evoked by hyperosmolarity in cultured astrocytes. J Neurosci Res 32: 86–92
Sănchez-Olea R, Morăn J, Martinez A, Pasantes-Morales H (1993a) Volume-activated Rb+ transport in astrocytes in culture. Am J Physiol 264: C836-C842
Sănchez-Olea R, Pena C, Morăn J, Pasantes-Morales H (1993b) Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl transport in cultured astrocytes. Neurosci Lett 156: 141–144
Sănchez-Olea R, Morales-Mulia M, Morăn J, Pasantes-Morales H (1995a) Inhibition by polyunsaturated fatty acids of regulatory volume decrease and osmolyte fluxes in astrocytes in culture. Am J Physiol 269: C96-C192
Sănchez-Olea R, Morales-Mulia M, Morăn J, Pasantes-Morales H (1995b) Inhibition by dihydropyridines of regulatory volume decrease and osmolyte fluxes in cultured astrocytes is unrelated to extracellular calcium. Neurosci Lett 193: 165–168
Sánchez-Olea R, Morales-Mulia M, Carcia O, Pasantes-Morales H (1996) Chloride channel blockers and polyunsaturated fatty acids similarly inhibit the volume activated pathways for Cl- and taurine in cultured cerebellar granule neurons. Am J Physiol (in press)
Schousboe A, Pasantes-Morales H (1989) Potassium-stimulated release of IH-taurine from cultured GABAergic and glutamatergic neurons. J Neurochem 53: 1309–1315
Schousboe A, Moran J, Pasantes-Morales H (1990) Potassium-stimulated release of taurine from cultured cerebellar granule neurons is associated with cell swelling. J Neurosci Res 27: 71–77
Schousboe A, Sănchez-Olea R, Morăn J, Pasantes-Morales H (1991) Hyposmolarity induced taurine release in cerebellar granule clls is associated with diffusion and not with high affinity transport. J Neurosci Res 30: 661–665
Solis JM, Herranz AS, Herreras O, Lerma J, del Rio RM (1988) Does taurine act as an osmoregulatory substance in the rat brain. Neurosci Lett 91: 53–58
Strange K, Morrison R, Shrode L, Putnanm R (1993) Mechanism and regulation of swelling-activated inositol efflux in brain glial cells. Am J Physiol 265: C244-C256
Strange K, Emma F, Paredes A, Morrison R (1994) Osmoregulatory changes in myoinositol content and Na/myoinositol cotransport in rat cortical astrocytes. Glia 12: 3543
Thurston JH, Hauhart RD, Dirco JA (1980) Taurine: a role in osmotic regulation in mammalian brain and possible clinical significance. Life Sci 26: 1561–1568
Thurston JH, Hauhart RD, Nelson JS (1987) Adaptive decreases in amino acids (taurine in particular), creatine and electrolytes, prevent cerebral edema in chronically hyponatremic mice: rapid correction causes dehydration and shrinkage of brain. Metab Brain Dis 2: 223–241
Trachtman H (1992) Cell volume regulation: a review of cerebral adaptive mechanisms and implications for clinical treatment of osmolal disturbances: II. Pediatr Nephrol 6: 104–112
Valverde MA, Diaz M, Sepdlveda FV, Gill DR, Hyde SC, Higgins CF (1992) Volumeregulated chloride channels associated with the human multidrug resistance P glycoprotein. Nature 355: 830–833
Verbalis JG, Gullans SR (1991) Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res 567: 274–282
Wade JV, Olsson JP, Samson FE, Nelson SR, Pazdernik TL (1988) A possible role for taurine in osmoregulation within the brain. J Neurochem 45: 335–344
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pasantes-Morales, H., Schousboe, A. Role of taurine in osmoregulation in brain cells: Mechanisms and functional implications. Amino Acids 12, 281–292 (1997). https://doi.org/10.1007/BF01373008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01373008