Summary
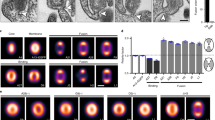
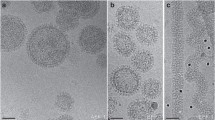
Fusion (fusion from within) of polarized MDCK monolayer cells grown on porous membranes was examined after infection with Sendai viruses. Wild-type virus, that buds at the apical membrane domain, did not induce cell fusion even when the F glycoprotein expressed at the apical domain was activated with trypsin. On the other hand, a protease activation mutant, F 1-R, with F protein in the activated form and that buds bipolarly at the apical and basolateral domains, caused syncytia formation in the absence of exogenous protease. Anti-Sendai virus antibodies added to the basolateral side, but not at the apical side, inhibited cell fusion induced by F 1-R. In addition, T-9, a mutant with bipolar budding phenotype of F 1-R but with an uncleavable F protein phenotype like wild-type virus, induced cell fusion exclusively when trypsin was added to the basolateral medium. By electron microscopy, cell-to-cell fusion was shown to occur at the lateral domain of the plasma membrane. These results indicate that in addition to proteolytic activation of the F protein, basolateral expression of Sendai virus envelope glycoproteins is required to induce cell fusion.
Similar content being viewed by others
References
Bratt MA, Gallaher WR (1969) Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci USA 64: 536–540
Caplan M, Matlin KS (1989) Sorting of membrane and secretory proteins in polarized epithelial cells. Mod Cell Biol 8: 71–127
Cerijido M, Contreras RG, Gonzalez-Mariscal L (1989) Development and alteration of polarity. Annu Rev Physiol 51: 785–795
Compans RW, Srinivas RV (1991) Protein sorting in polarized epithelial cells. Curr Top Microbiol Immunol 170: 141–181
Homma M (1972) Trypsin action of the growth of Sendai virus in tissue culture cells. II. Restoration of the hemolytic activity of L cell-borne Sendai virus by trypsin. J Virol 9: 829–835
Homma M, Ohuchi M (1973) Trypsin action on the growth of Sendai virus in tissue culture cells. III. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol 12: 1457–1465
Homma M, Tamagawa S (1973) Restoration of the fusion activity of L cell-borne Sendai virus by trypsin. J Gen Virol 19: 423–426
Hosaka Y, Shimizu K (1977) Cell fusion by Sendai virus. In: Poste G, Nicolson GL (eds) Virus infection and the cell surface. North-Holland, Amsterdam, pp 129–155
Kim J, Adachi T, Yoneda Y, Okada Y (1990) Fusion of Madin-Darby canine kidney cells by HVJ (Sendai virus): absence of direct association of viral particles with the site of membrane fusion. Eur J Cell Biol 51: 128–134
Kohn A (1965) Polykaryocytosis induced by Newcastle disease virus in monolayers of animal cells. Virology 26: 228–245
Merz DC, Scheid A, Choppin PW (1980) Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med 151: 275–288
Middleton Y, Tashiro M, Thai T, Oh J, Seymour J, Pritzer E, Klenk H-D, Rott R, Seto JT (1990) Nucleotide sequence analyses of the genes encoding the HN, M, NP, P, and L proteins of two host range mutants of Sendai virus. Virology 176: 656–657
Mostov KE, Simister NE (1985) Transcytosis. Cell 43: 389–390
Muramatsu M, Homma M (1980) Trypsin action of the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken eggs. Microbiol Immunol 24: 113–122
Nayak DP, Jabbar MA (1989) Structural domains and organizational conformation involved in the sorting and transport of influenza virus transmembrane proteins. Annu Rev Microbiol 43: 465–501
Nelson WJ (1989) Development and maintenance of epithelial cell polarity: a role for the submembranous cytoskeleton. Mod Cell Biol 8: 3–42
Ohnishi S (1988) Fusion of viral envelopes and cellular membranes. Curr Top Membr Transp 32: 257–296
Okada Y (1988) Sendai virus-mediated cell fusion. Curr Top Membr Transp 32: 297–336
Rodriguez-Boulan E, Nelson WJ (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245: 718–725
Rodriguez-Boulan E, Sabatini DD (1978) Asymmetric budding of viruses in epithelial monolayers: a model system for study of epithelial polarity. Proc Natl Acad Sci USA 75: 5071–5075
Scheid A, Choppin PW (1974) Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity by proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology 57: 475–490
Simons K, Fuller SD (1985) Cell surface polarity in epithelia. Annu Rev Cell Biol 1: 248–288
Simons K, Wandinger-Ness A (1990) Polarized sorting in epithelia. Cell 62: 207–210
Tashiro M, Pritzer E, Khoshnan MA, Yamakawa M, Kuroda K, Klenk H-D, Rott R, Seto JT (1988) Characterization of a pantropic variant of Sendai virus derived from a host-range mutant. Virology 165: 577–583
Tashiro M, Yamakawa M, Tobita K, Klenk H-D, Rott R, Seto JT (1990) Organ tropism of Sendai virus in mice: proteolytic activation of the fusion glycoprotein in mouse organs and budding site at the bronchial epithelium. J Virol 64: 3627–3634
Tashiro M, Yamakawa M, Tobita K, Seto JT, Klenk H-D, Rott R (1990) Altered budding site of a pantropic mutant of Sendai virus, F 1-R, in polarized epithelial cells. J Virol 64: 4672–4677
Tashiro M, James I, Karri S, Wahn K, Tobita K, Klenk H-D, Rott R, Seto JT (1991) Pneumotropic revertants derived from a pantropic mutant, F 1-R, of Sendai virus. Virology 184: 227–234
Tashiro M, Seto JT, Choosakul S, Yamakawa M, Klenk H-D, Rott R (1992) Budding site of Sendai virus in polarized epithelial cells is one of the determinants for tropism and pathogenicity in mice. Virology 187 (in press)
White J, Kielian M, Helenius A (1983) Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys 16: 151–195
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tashiro, M., Yamakawa, M., Tobita, K. et al. Significance of basolateral domain of polarized MDCK cells for Sendai virus-induced cell fusion. Archives of Virology 125, 129–139 (1992). https://doi.org/10.1007/BF01309633
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309633