Abstract
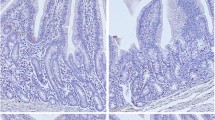
Ten crossbred wether lambs were fed once daily an oat diet that contained 3103 μg of retinyl palmitate (control) and were supplemented with 55,000 μg of retinyl palmitate orally once every two weeks. Twenty lambs were fed the same oat diet without retinyl palmitate supplements (A-depleted). After being fed the A-depleted diet for 28 weeks, 10 A-depleted lambs were repleted by feeding the control diet and oral supplementation of retinyl palmitate for eight weeks. The A-depleted lambs had serum vitamin A concentrations indicative of vitamin A deficiency, which was supported by very low liver vitamin A concentrations. Light microscopic and ultrastructural examinations revealed that alterations occurred at 50% and 75% of the small intestine length in A-depleted lambs only. Sawtooth configuration of the intestinal epithelium was a distinctive histologic feature. Consistent ultrastructural alterations were vesicular microvillar degeneration and disruption of the capillary endothelium. These results suggests that A-depleted diets have a detrimental effect on the small intestinal epithelium of lambs. Vitamin A repletion appears to minimize the detrimental effects.
Similar content being viewed by others
References
Schoeff L: Vitamin A. Am J Med Technol 49:447–452, 1983
Zile MH, Cullum ME: The function of vitamin A: Current concepts. Proc Soc Exp Biol Med 172:139–152, 1983
Bondi A, Sklan D: Vitamin A and carotene in animal nutrition. Prog Food Nutr Sci 8:165–191, 1984
Wolf G: Multiple functions of vitamin A. Physiol Rev 64:873–937, 1984
Sklan D: Vitamin A in human nutrition. Prog Food Nutr Sci 11:39–55, 1987
McDowell LR: Vitamins in Animal Nutrition. Comparative Aspects to Human Nutrition. San Diego, California, Academic Press, 1989
DeLuca L, Wolf G: Mechanism of action of vitamin A in differentiation of mucus-secreting epithelia. J Agric Food Chem 20:474–476, 1972
Wong YC, Buck RC: An electron microscopic study of metaplasia of the rat tracheal epithelium in vitamin A deficiency. Lab Invest 24:55–66, 1971
Hicks RM: Hyperplasia and cornification of the transitional epithelium in vitamin A-deficient rat. J Ultrastruct Res 22:206–230, 1968
Jungherr EL, Helmboldt CF, Eaton HD: Parotid gland lesions in experimental bovine vitamin A deficiency. J Dairy Sci 33:666–675, 1950
Eaton HD, Helmboldt CF, Jungherr EL, Carpenter CA: Effect of vitamin A depletion on live weight, plasma and liver levels of vitamin A and microanatomy in young dairy calves. J Dairy Sci 34:386–395, 1951
Helmboldt CF, Jungherr EL, Eaton HD, Moore LA: The pathology of experimental hypovitaminosis A in young dairy animals. Am J Vet Res 14:343–354, 1953
Hays KC, McCombs HL, Faherty TP: The fine structure of vitamin A deficiency. I. Parotid duct metaplasia. Lab Invest 22:81–89, 1970
Nielsen SW, Mills JHL, Rousseau JE, Woelfel CG: Parotid duct metaplasia in marginal bovine vitamin A deficiency. Am J Vet Res 27:223–233, 1966
De Luca L, Little EP, Wolf G: Vitamin A and protein synthesis by rat intestinal mucosa. J Biol Chem 244:701–708, 1969
Zile M, Bunge EC, DeLuca HF: Effect of vitamin A deficiency on intestinal cell proliferation in the rat. J Nutr 107:552–560, 1977
Thorp WTS, Keener HA, Bechdel SI, Guerrant NB: Observations on the pathology of dairy calves on low vitamin A diets. Am J Vet Res 3:27–31, 1942
Rojanapo W, Lamb AJ, Olson JA: The prevalence, metabolism, and migration of goblet cells in rat intestine following the induction of rapid, synchronous vitamin A deficiency. J Nutr 110:178–188 1980
Olson JA, Rojanapo W, Lamb AJ: The effect of vitamin A status on the differentiation and function of goblet cells in the rat intestine. Ann NY Acad Sci 359:181–191, 1981
Hart GH, Guilbert HR: Symptomatology of vitamin-A deficiency in domestic animals. J Am Vest Assoc 14:193–200, 1937
Eaton HD, Matterson LD, Decker L, Helmboldt CF, Jungherr EL: Intravenous and oral administration of an aqueous suspension of carotene to calves depleted of their vitamin A stores. J Dairy Sci 34:1073–1080, 1951
United States Department of Health and Human Services. Guide for the care and use of laboratory animals 1985. National Institutes of Health Publication No. 85-23.
Kimble MS: The photoelectric determination of vitamin A and carotene in human plasma. J Lab Clin Med 24:1055–1068, 1939
Dugan RE, Frigui NA, Seibert JM: Colorimetric determination of vitamin A and its derivatives. Anal Chem 36:114–117, 1964
Gallup WD, Hoefer JA: Determination of vitamin A in liver. Ind Eng Chem 18:288–290, 1946
Pfeiffer CJ, Pfeiffer DC, Misra HP: Enteric serosal surface in the piglet: A scanning and transmission electron microscopic study of the mesothelium. J Submicrosc Cytol 19:237–246, 1987
Eaton HD, Lucas JJ, Nielsen SW: Association of plasma or liver vitamin A concentrations with the occurrence of parotid duct metaplasia or of ocular papilledema in Holstein male calves. J Dairy Sci 53:1775–1779, 1970
May BJ, Calhoun MC, Engdahl GR: A re-evaluation of the minimum vitamin A requirement of growing-finishing lambs. J Anim Sci 65:1626–1632, 1987
DeLuca L, Schumacher M, Wolf G: Biosynthesis of a fucose-containing glycopeptide from rat small intestine in normal and vitamin A-deficient conditions. J Biol Chem 245:4551–4558, 1970
Sirisinha S, Darip MD, Moonkarndi P, Ongsakul M, Lamb AJ: Impaired local immune response in vitamin A-deficient rats. Clin Exp Immunol 40:127–135, 1980
Gregory MW, Catchpole J: Ovine coccidiosis: Heavy infection in young lambs increases resistance without causing disease. Vet Rec 124:458–461, 1989
Ruff MD, Fuller HL: Some mechanisms of reduction of carotenoid levels in chickens infected withEimeria acervulina orE. tenella. J Nutr 105:1447–1456, 1975
Erasmus J, Scott ML, Levine PP: A relationship between coccidiosis and vitamin A nutrition in chickens. Poult Sci 39:565–575, 1960
Author information
Authors and Affiliations
Additional information
This research was supported by the State Agriculture Experiment Station (USDA Hatch Funds) Virginia Tech, Blacksburg, Virginia 24061.
Rights and permissions
About this article
Cite this article
Holland, R.E., Pfeiffer, C.J., Bruns, N.J. et al. Morphologic alterations in small intestinal epithelium of lambs fed vitamin A-depleted diet. Digest Dis Sci 38, 333–343 (1993). https://doi.org/10.1007/BF01307553
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01307553