Summary
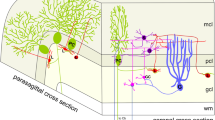
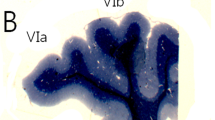
In the molecular layer of the mouse cerebellum, the histochemical activity of the adenosine-producing ectoenzyme 5′-nucleotidase discloses a parasagittal pattern of alternating enzyme-rich and enzyme-poor bands. In the rat, 5′-nucleotidase activity transiently labels cerebellar synapses during postnatal development and shifts later on towards an exclusive glial location in the molecular layer. We therefore asked whether different ultrastructural expression of 5′-nucleotidase would account for the light microscopic pattern seen in the adult mouse cerebellum. Using an enzyme cytochemical method, we localized 5′-nucleotidase activity on the glial cells and at the main types of asymmetrical synapses in the developing and mature cerebellum of the mouse. The percentage of labelled synapses increased until adulthood within the 5′-nucleotidasepositive bands. Here, the vast majority (86%) of the synapses were labelled against only 27% within the negative bands in the adult. Thus, 5′-nucleotidase appears as a marker of glia and of Purkinje cell synapses across cerebellar compartments. Changes in purinergic neuromodulation and/or cell adhesion mediated by 5′-nucleotidase across bands might participate in the functional differentiation of the cerebellar parasagittal subsets.
Similar content being viewed by others
References
Bailly, Y., Schoen, S. W., Delhaye-Bouchaud, N., Kreutzberg, G. W. &Mariani, J. (1990) Localisation synaptique de l'activité 5′-nucléotidase dans le cortex cérébelleux du rat adulte irradié aux rayons-X après la naissance.Comptes Rendus de l'Académie des Sciences (Paris) 311, 487–93.
Bailly, Y., Schoen, S. W., Delhaye-Bouchaud, N., Kreutzberg, G. W. &Mariani, J. (1993) Synaptic 5′- nucleotidase activity in the cerebellum of normal and reeler mouse.Neuroscience Abstracts 19, 648.
Boegman, R., Parent, A. &Hawkes, R. (1988) Zonation in the rat cerebellar cortex: patches of high acetyl-cholinesterase activity in the granular layer are congruent with Purkinje cell compartments.Brain Research 448, 237–51.
Brochu, G., Maler, L. &Hawkes, R. (1990) Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum.Journal of Comparative Neurology 291, 538–52.
Codogno, P., Doyenette-Moyne, M. A., Aubery, M., Dieckhoff, J., Lietzke, R. &Mannherz, H. G. (1988) Polyclonal and monoclonal antibodies against chicken gizzard 5′-nucleotidase inhibit the spreading process of chicken embryonic fibroblasts on laminin substratum.Experimental Cell Research 174, 344–54.
Do, K. Q., Vollenweider, F. &Cuenod, M. (1988) Endogenous adenosine and excitatory amino acid release in rat cerebellum.European Journal of Neuroscience (Suppl. 1), 106.
Drejer, J., Frandsen, A., Honore, T. &Schousboe, A. (1987) Adenosine inhibits glutamate stimulated (3H)D-aspartate release from cerebellar granule cells.Neurochemistry International 11, 77–81.
Dunwiddie, T. V. (1985) The physiological role of adenosine in the central nervous system.International Review of Neurobiology 27, 63–139.
Eisenman, L. M. (1988) Histochemical localization of 5′- nucleotidase in thereeler mutant mouse.Neuroscience Letters 94, 70–5.
Eisenman, L. M. &Hawkes, R. (1989) 5′-nucleotidase and the mabQ113 antigen share a common distribution in the cerebellar cortex of the mouse.Neuroscience 31, 231–5.
Eisenman, L. M. &Hawkes, R. (1993) Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography.Journal of Comparative Neurology 335, 586–605.
Fischer, M. &Mullen, R. J. (1988) Neuronal influence on glial enzyme expression: evidence from chimeric mouse cerebellum.Neuron 1, 151–7.
Fischer, M., Trimmer, P. &Ruthel, G. (1993) Bergmann glia require continuous association with Purkinje cells for normal phenotype expression.Glia 8, 172–82.
Geiger, J. D., Labella, F. S. &Nagy, J. (1984) Ontogenesis of adenosine receptors in the central nervous system of the rat.Developmental Brain Research 13, 97–104.
Gravel, C. &Hawkes, R. (1990) Parasagittal organization of the rat cerebellar cortex: direct comparison of Purkinje cell compartments and the organization of the spinocerebellar projection.Journal of Comparative Neurology 291, 79–102.
Gravel, C., Eisenman, L., Sasseville, R. &Hawkes, R. (1987) Parasagittal organization of the rat cerebellar cortex: direct correlation between antigenic Purkinje cell bands revealed by mabQ113 and the organization of the olivocerebellar projection.Journal of Comparative Neurology 265, 294–310.
Hamori, J. &Szentagothai, J. (1980) Lack of evidence of synaptic contacts by climbing fibre collaterals to basket and stellate cells in developing rat cerebellar cortex.Brain Research 186, 454–7.
Hardonk, M. J. &De Boer, H. G. A. (1968) 5′- Nucleotidase: III. Determinations of 5′-nucleotidase isoenzymes in tissues of rat and mouse.Histochemie 12, 29–41.
Hawkes, R. (1992) Antigenic markers of cerebellar modules in the adult mouse.Biochemical Society Transactions 20, 391–5.
Hawkes, R. &Leclerc, N. (1987) Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by monoclonal anti-Purkinje cell antibody mabQ113.Journal of Comparative Neurology 256, 29–41.
Hawkes, R., Colonnier, M. &Leclerc, N. (1985) Monoclonal antibodies reveal sagittal banding in the rodent cerebellar cortex.Brain Research 333, 359–65.
Hess, D. &Hess, A. (1986) 5′-Nucleotidase of cerebellar molecular layer: reduction in Purkinje cell-deficient mutant mice.Developmental Brain Research 29, 93–100.
Heymann, D., Reddington, M. &Kreutzberg, G. W. (1984) Subcellular localization of 5′-nucleotidase in rat brain.Journal of Neurochemistry 43, 971–8.
Kocsis, J. D., Eng, D. L. &Bhizitkul, R. B. (1984) Adenosine selectively blocks parallel-fiber-mediated synaptic potentials in rat cerebellar cortex.Proceedings of the National Academy of Sciences (USA) 81, 6531–4.
Kreutzberg, G. W., Barron, K. D. &Schubert, P. (1978) Cytochemical localization of 5′-nucleotidase in glial plasma membranes.Brain Research 158, 247–57.
Kreutzberg, G. W., Heymann, D. &Reddington, M. (1986) 5′-Nucleotidase in the nervouse system. InCellular Biology of Ectoenzymes (edited byKreutzberg, G. W., Reddington, M. &Zimmermann, H.) pp. 147–64. Berlin: Springer.
Kuchler, S., Zanetta, J.-P., Bon, S., Zaepfel, M., Massoulie, J. &Vincendon, G. (1991) Expression and localization in the developing cerebellum of the carbohydrate epitopes revealed by ELEC-39, and IgM monoclonal antibody related to HNK-1.Neuroscience 41, 551–62.
Larramendi, L. M. &Victor, T. (1967) Synapses on the Purkinje cell spines in the mouse. An electronmicroscopic study.Brain Research 5, 15–30.
Leclerc, N., Dore, L., Parent, A. &Hawkes, R. (1990) The compartmentation of the monkey and rat cerebellar cortex: zebrin I and cytochrome oxidase.Brain Research 506, 70–8.
Leclerc, N., Schwarting, G., Herrup, K., Hawkes, R. &Yamamoto, M. (1992) Compartmentation in mammalian cerebellum: zebrin II and P-path antibodies define three classes of sagittally organized bands of Purkinje cells.Proceedings of the National Academy of Sciences (USA) 89, 5006–10.
Marani, E. (1982)Topographic Enzyme Histochemistry of the Mammalian Cerebellum: 5′-Nucleotidase and Acetylcholinesterase. Thesis, University of Leiden, The Netherlands.
Marani, E. &Voogd, J. (1977) An acetylcholinesterase band pattern in the molecular layer of the cat cerebellum.Journal of Anatomy 124, 335–45.
Mugnaini, E., Floris, A. &Wright-Goss, M. (1994) Extraordinary synapses of the unipolar brush cell: an electron microscopic study in the rat cerebellum.Synapse 16, 284–311.
Ottersen, O. P. (1993) Neurotransmitters in the cerebellum.Revue Neurologique 149, 629–36.
Palay, S. L. &Chan-Palay, V. (1974)Cerebellar Cortex. Cytology and Organization. Berlin: Springer.
Prince, D. A. &Stevens, C. F. (1992) Adenosine decreases neurotransmitter release at central synapses.Proceedings of the National Academy of Sciences (USA) 89, 8586–90.
Richardson, P. J., Brown, S. J., Bailyes, E. M. &Luzio, J. P. (1987) Ectoenzymes control adenosine modulation of immunoisolated cholinergic synapses.Nature 327, 232–4.
Ross, C. A., Bredt, D. &Snyder, S. (1990) Messenger molecules in the cerebellum.Trends in Neurosciences 13, 216–22.
Schoen, S. W. &Graybiel, A. M. (1993) Species-specific patterns of glycoprotein expression in the developing rodent caudoputamen: association of 5′-nucleotidase activity with dopamine islands and striosomes in rat, but with extrastriosomal matrix in mouse.Journal of Comparative Neurology 333, 578–96.
Schoen, S. W. &Kreutzberg, G. W. (1994) Synaptic 5′- nucleotidase activity reflects lesion-induced sprouting within the adult rat dentate gyrus.Experimental Neurology 127, 106–18.
Schoen, S. W. &Kreutzberg, G. W. (1995) Evidence that 5′-nucleotidase is associated with malleable synapses — an enzyme cytochemical investigation of the olfactory bulb of adult rats.Neuroscience 65, 37–50.
Schoen, S. W., Graeber, M. B., Reddington, M. &Kreutzberg, G. W. (1987) Light and electron microscopical immunocytochemistry of 5′-nucleotidase in rat cerebellum.Histochemistry 87, 107–13.
Schoen, S. W., Graeber, M. B., Toth, L. &Kreutzberg, G. W. (1988) 5′-nucleotidase in postnatal ontogeny of rat cerebellum: a marker for migrating nerve cells?Developmental Brain Research 39, 125–36.
Schoen, S. W., Graeber, M. B., Toth, L. &Kreutzberg, G. W. (1991) Synaptic 5′-nucleotidase is transient and indicative of climbing fiber plasticity during the postnatal development of rat cerebellum.Developmental Brain Research 61, 125–38.
Schoen, S. W., Kreutzberg, G. W. &Singer, W. (1993) Cytochemical redistribution of 5′-nucleotidase in the developing visual cortex.European Journal of Neuroscience 5, 210–22.
Scott, T. G. (1963) A unique pattern of localization within the cerebellum.Nature 200, 793.
Scott, T. G. (1964) A unique pattern of localization within the cerebellum of the mouse.Journal of Comparative Neurology 122, 1–8.
Scott, T. G. (1965) The specificity of 5′-nucleotidase in the brain of the mouse.Journal of Histochemistry and Cytochemistry 13, 657–67.
Stochaj, U. &Mannherz, H. G. (1992) Chicken gizzard 5′-nucleotidase function as a binding protein for the laminin/nidogen complex.European Journal of Cell Biology 59, 364–72.
Stochaj, U., Dieckhoff, J., Mollenhauer, J., Cramer, M. &Mannherz, H. G. (1989) Evidence for the direct interaction of chicken gizzard 5′-nucleotidase with laminin and fibronectin.Biochimica et Biophysica Acta 992, 385–92.
Stochaj, U., Richter, H. &Mannherz, H. G. (1990) Chicken gizzard 5′-nucleotidase is a receptor for the extracellular matrix component fibronectin.European Journal of Cell Biology 51, 335–8.
Tano, D., Napieralsky, J. A., Eisenman, L. M., Messer, A., Plummer, J. &Hawkes, R. (1992) Novel developmental boundary in the cerebellum revealed by zebrin expression in the lurcher (Lc/+) mutant mouse.Journal of Comparative Neurology 323, 128–36.
Vogel, M., Zimmermann, H. &Singer, W. (1993) Transient association of the HNK-1 epitope with 5′- nucleotidase during development of the cat visual cortex.European Journal of Neuroscience 5, 1423–5.
Vogel, M., Kowalewski, H. J., Zimmermann, H., Janetzko, A., Margolis, R. U. &Wollny, H.-E. (1991) Association of the HNK-1 epitope with 5′-nucleotidase fromTorpedo marmorata (electric ray) electric organ.Biochemical Journal 278, 199–202.
Wassef, M., Cholley, B., Heizmann, C. W. &Sotelo, C. (1992) Development of the olivo-cerebellar projection in the rat: II. Matching of the developmental compartmentations of the cerebellum and inferior olive through the projection map.Journal of Comparative Neurology 323, 537–50.
Wojcik, W. J. &Neff, N. H. (1983) Adenosine A1 receptors are associated with cerebellar granule cells.Journal of Neurochemistry 41, 759–63.
Zimmermann, H. (1992) 5′-Nucloetidase: molecular structure and functional aspects.Biochemical Journal 285, 345–65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bailly, Y., Schoen, S.W., Delhaye-Bouchaud, N. et al. 5′-Nucleotidase activity as a synaptic marker of parasagittal compartmentation in the mouse cerebellum. J Neurocytol 24, 879–890 (1995). https://doi.org/10.1007/BF01179986
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01179986