Abstract
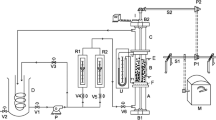
The distribution of current density in a cylindrical electrochemical reactor was determined experimentally, a thin Pt wire, 0.2 mm diameter and 500–600 mm long, being employed as central electrode. In these investigations two methods are used: (i) a reactor with a segmented counterelectrode; in this case, measurements of the current in each ring of the counterelectrode were made; (ii) a reactor with a bi-electrode probe; in this case, the distribution was obtained by measuring the ohmic drop in the solution phase, with the probe being positioned at different heights. A mathematical model to represent such reactors was developed assuming an axially constant electrolyte potential. The experimental and theoretical values are compared in order to determine the predictive suitability of the proposed model. Both the error in predicting the feeder overvoltage and a statistical parameter\((\bar \delta _r )\) denote the agreement between the computed and the measured current density distributions. The parameters acting upon the current distribution were lumped in a single dimensionless variable, the so-called modified Wagner number, used to determine the applicability range of the proposed model. It was concluded that when this number exceeds 15×10−3, for concentrated solutions, the model can be used to design this type of reaction.
Similar content being viewed by others
Abbreviations
- A 5 :
-
surface area per unit volume of electrode
- b :
-
constant defined by Equation 12 (V−1)
- B :
-
integration constant defined by Equation 15
- E :
-
error percentage
- E 0 :
-
reversible electrode potential (V)
- i :
-
current density (A cm−2)
- \(\bar i\) :
-
average current density at Δx (A cm−2)
- i 0 :
-
exchange current density (A cm−2)
- I :
-
total current (A)
- L :
-
electrode length (cm)
- N :
-
number of experimental values in Equation 28
- r :
-
radius (cm)
- r r :
-
radial position of the reference electrode (cm)
- R m :
-
resistance of the metal phase (Ω)
- R p :
-
polarization resistance (Ω)
- RT/F :
-
constant (0.0257V at 25°C) (V)
- Wa * :
-
modified Wagner number
- x :
-
axial coordinate (cm)
- x c :
-
charge transfer coefficient
- γ:
-
electrode effectiveness factor
- \(\bar \delta _r \) :
-
parameter which evaluates the predictive ability of the model to determine current density distribution
- ΔV :
-
potential of the working electrode atx=0 with respect to the standard hydrogen electrode including the electrolyteiR drop (V)
- V e :
-
charge number of the electrode reaction
- η:
-
overvoltage (V)
- ϱ:
-
resistivity (Ω cm)
- π:
-
potential (V)
- e:
-
electrode
- exp:
-
experimental
- m:
-
metal phase
- s:
-
solution phase
- th:
-
theoretical
References
A. J. Bellamy and B. R. Simpson,Chem. Ind. (Lond.) (1981) 328.
A. Weisselberg and Staff,Trans. Electrochem. Soc. 90 (1946) 235.
C. W. Tobias and R. Wijsman,J. Electrochem. Soc. 100 (1953) 459.
W. W. Harvey,J. Electrochem. Soc. 109 (1962) 638.
S. Zaromb,J. Electrochem. Soc. 109 (1962) 912.
B. E. Conway, E. Gileadi and H. G. Oswin,Can. J. Chem. 41 (1963) 2447.
S. K. Rangarajan, M. J. Dignam and B. E. Conway,Can. J. Chem. 45 (1967) 422.
J. Wojtowicz, L. Laliberté and B. E. Conway,Electrochim. Acta 13 (1968) 361.
R. Alkire and R. Varjian,J. Electrochem. Soc. 121 (1974) 622.
A. Tvarusko,J. Electrochem. Soc. 121 (1974) 660.
P. M. Robertson,Electrochim. Acta 22 (1977) 411.
R. Hertwig and J. Breme,Chem. Techn. (Leipzig) 32 (1980) 294.
K. Scott,J. Appl. Electrochem. 13 (1983) 209.
C. Wagner,J. Electrochem. Soc. 98 (1951) 116.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bisang, J.M., Kreysa, G. Study of the effect of electrode resistance on current density distribution in cylindrical electrochemical reactors. J Appl Electrochem 18, 422–430 (1988). https://doi.org/10.1007/BF01093758
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01093758