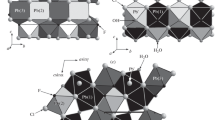
Summary
The crystal structure of a schröckingerite from Joachimsthal, NaCa3[UO2(CO3)3](SO4) F·10H2O, triclinic, space groupP1,a=9.634(1),b=9.635(1),c=14.391(2) Å, α-91.41(1), β=92.33(1), γ=120.26(1)°,V=1151 Å3,Z=2, has been determined by X-ray diffraction and refined toR=0.026 for 5451 reflections. The structure contains NaCa3[UO2(CO3)3] (SO4) F·6H2O layers built up from UO2(CO3) 4−3 anions, NaO3(H2O)3 octahedra, three kinds of CaO5F(H2O)2 polyhedra, Ca3F pyramids and Ca-bonded SO4 tetrahedra. These layers extend atz∼1/5 andz∼4/5 parallel to (001). They are linked parallel to c exclusively by hydrogen bonds, both directly as well as via interlayer H2O molecules. The structure shows a striking trigonal pseudosymmetry within the range 0.04<z<0.96. Atz∼0 these parts of the structure are dislocated relative to each other by a step of ∼1 Å parallel to [110]. Morphologic and optical properties of schröckingerite have been investigated in the light of the known crystal structure.
Zusammenfassung
Die Kristallstruktur eines Schröckingerits von Joachimsthal, NaCa3[UO2(CO3)3](SO4) F·10H2O, triklin, RaumgruppeP1,a=9,634(1),b=9,635(1),c=14,391(2) Å, α=91,41(1), β=92,33(1), γ=120,26(1)°,V=1151 Å3,Z=2, wurde mit Röntgenbeugung bestimmt und für 5451 Reflexe aufR=0.026 verfeinert. Die Kristallstruktur enthält NaCa3[UO2(CO3)3] (SO4)I·6H2O Schichten, die aus UO2(CO3) 4−3 . Anionen, NaO3(H2O)3-Oktaedern, drei Arten von CaO5F(H2O)2-Polyedern, Ca3 F-Pyramiden und an Ca gebundenen SO4-Tetraedern aufgebaut sind. Diese Schichten erstrecken sich inz∼1/5 undz∼4/5 parallel zu (001). Sie sind parallel zuc ausschließlich durch Wasserstoffbrücken verknüpft, und zwar sowohl direkt als auch indirekt durch zwischen den Schichten gelegene Wassermoleküle. Die Struktur zeigt im Bereich 0,04<z<0,96 eine ausgeprägte trigonale Pseudosymmetrie. Derartige Bereiche sind inz∼0 um etwa 1 Å parallel zu [110] stufenartig gegeneinander versetzt. Morphologische und optische Eigenschaften von Schröckingerit wurden im Licht der bekannten Kristallstruktur untersucht.
Similar content being viewed by others
References
Anderson, A., Chung Chieh, Irish, D. E., Tong, J.P.K., 1980: An X-ray crystallographic, Raman, and infrared spectral study of crystalline potassium uranyl carbonate, K4UO2(CO3)3. Can. J. Chem.58, 1651–1658.
Axelrod, J. M., Grimaldi, F. S., Milton, C., Murata, K. J., 1951: The uranium minerals from the Hillside Mine. Yavapai County, Arizona. Amer. Min.36, 1–22.
Bloss, F. D., 1981: The spindle stage-principles and practise. Cambridge: Cambridge University Press.
Cejka, J., Urbanec, Z., 1980: Uranium secondary minerals in the collection of the National Museum in Prague. VI. Schröckingerit. Časopis Národního Muzea-řada přírodovědná149, 60–68. (In Czech.)
Coda, A., Della Giusta, A., Tazzoli, V., 1981: The structure of synthetic andersonite, Na2Ca [UO2(CO3)3]·xH2O (x∼5.6). Acta Cryst.B37, 1496–1500.
Einspahr, H., Bugg, C. E., 1980: The geometry of calcium-water interactions in crystalline hydrates. Acta Cryst.B36, 264–271.
Frondel, C., 1958: Systematic mineralogy of uranium and thorium, pp. 121–126. U.S. Geological Survey Bulletin 1064, Washington: U.S. Govt. Print. Office.
Graziani, R., Bombieri, G., Forsellini, E., 1972: Crystal structure of tetra-ammonium uranyl tricarbonate. J. Chem. Soc. Dalton Trans.1972, 2059–2061.
Hauser, J., Wenk, H.-R., 1976: Optical properties of composite crystals (submicroscopic domains, exsolution lamellae, solid solutions). Z. Krist.143, 188–219.
Hurlbut, C. S., Jr., 1954: Uranium minerals. XV. Schroeckingerite from Argentina and Utah. Amer. Min.39, 901–907.
Jaffe, H. W., Sherwood, A. M., Peterson, M. J., 1948: New data on schroeckingerite. Amer. Min.33, 152–157.
Mazzi, F., Rinaldi, F., 1961: La struttura cristallina del K3Na(UO2)(CO3)3. Period. Min.30, 1–20.
Mereiter, K., 1982: The crystal structure of liebigite, Ca2UO2(CO3)3·∼11H2O. Tschermaks Min. Petr. Mitt.30, 277–288.
Mereiter, K., 1983: The crystal structure of schroeckingerite, NaCa3UO2(CO3)3SO4F·10H2O. Eighth European Cryst. Meeting, Liege, Belgium 1983, Abstracts p. 108.
—, 1984: The crystal structure of albrechtschraufite, MgCa4F2(UO2)2(CO3)6·17H2O. Acta Cryst.A40, Supplement, C-247.
—,Preisinger, A., 1984: Bestimmung der optischen Orientierung mittels Spindeltisch und Röntgen-Vierkreisdiffraktometer. Fortschr. Min.62, Beiheft 1, 53.
Novacek, R., 1939: The identity of dakeite and schroeckingerite. Amer. Min.24, 317–323.
Palache, C., Berman, H., Frondel, C., 1951: Dana's System of Mineralogy, 7th ed., Vol. 1, pp. 1–15, New York and London: Wiley and Chapman.
Ross, V., 1955: Studies of uranium minerals (XVII). Synthetic schröckingerite. Amer. Min.40, 515–519.
Schrauf, A., 1873: Schröckingerit, ein neues Mineral von Joachimsthal. Tschermaks Min. Petr. Mitt.3, 137–138.
Shannon, R. D., Prewitt, C. T., 1969: Effective ionic radii in oxides and fluorides. Acta Cryst.B25, 925–946.
Sheldrick, G. M., 1976: SHELX 76, program for crystal structure determination. Univ. of Cambridge.
Sheridan, D. M., Maxwell, C. H., Collier, J. T., 1962: Geology of the Lost Creek schroeckingerite deposits, Sweetwater County, Wyoming. U.S. Geol. Surv. Bull.1087-J, pp. 391–478.
Smith, D. K., 1959. An X-ray crystallographic study of schroeckingerite and its dehydration product. Amer. Min.44, 1020–1025.
Sudarsanan, K., Young, R. A., 1972: Structure of strontium hydroxide phosphate Sr5(PO4)3 (OH). Acta Cryst.B28, 3668–3670.
—,Mackie, P. E., Young, R. A., 1972: Comparison of synthetic and mineral fluorapatite, Ca5(PO4)3F, in crystallographic detail. Mat. Res. Bull.7, 1331–1338.
Walenta, K., 1972: Grimselit, ein neues Kalium-Natrium-Uranylkarbonat aus dem Grimselgebiet (Oberhasli, Kanton Bern, Schweiz). Schweiz. Min. Petr. Mitt.52, 93–108.
Wuensch, B. J., 1972: Sulfur, crystal chemistry. In: Handbook of Geochemistry (Wedepohl, K. H., ed.). Berlin-Heidelberg-New York: Springer-Verlag.
Zemann, J., 1981: Zur Stereochemie der Karbonate. Fortschr. Min.59, 95–116.
Author information
Authors and Affiliations
Additional information
With 6 Figures
Rights and permissions
About this article
Cite this article
Mereiter, K. Crystal structure and crystallographic properties of a Schröckingerite from Joachimsthal. TMPM Tschermaks Petr. Mitt. 35, 1–18 (1986). https://doi.org/10.1007/BF01081914
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01081914