Abstract
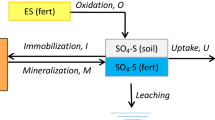
A mathematical model was developed to predict the release of sulfate from elemental S (S0) and gypsum in single superphosphate. The release algorithm is based on the observation that release is linearly related to particle surface area. Release rates under various conditions could then be described by the change in radius for each time increment, which allows easier comparison of release rates between different particle sizes. A model based on spherical particles was found to be adequate in accounting for the range of particle shapes found in crushed agricultural S0. Release rates calculated from experimental data range from 0.07 to 0.45µm/d depending on environmental conditions.
Equations for incorporating the effects of environmental variables and the release of S from S0 and from the gypsum component of single superphosphate (SSP) were developed from the literature, and were incorporated within a larger model of S cycling. The model predicted that after 72 days, 99% of the S in SSP would have been released, compared to a release after one year of 54% of the S in sulfur-fortified superphosphate, and 23% of that in crushed agricultural grade S0. The model provides a means of assessing the effect of the particle size of S0 on release rates and should allow the formulation of fertilizers that supply S at a rate closer to the rate of plant uptake.
Similar content being viewed by others
References
Barrow NJ (1971) Slowly available fertilizers in southwestern Australia. 1. Elemental sulfur. Aust J Exp Agric Anim Husb 11: 211–216
Boswell CC, Owers WR, Swanney B and Rothbaum HP (1988) Sulfur/sodium bentonite mixtures as sulfur fertilizers. 1. The effects of S/Na-bentonite ratios on the rate of dispersion and particle size distribution of elemental sulfur dispersed from laboratory-produced prills. Fert Res 15: 13–31
Brunauer S, Emmett PH and Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 59: 309–319
Chopra SL and Kanwa JS (1968) Effect of some factors on the transformation of elemental sulfur in soils. Indian Soc Soil Sci J 16: 83–88
Fawzi Abed, MAH (1976) Rate of elemental sulfur oxidation in some soils of Egypt as affected by the salinity level, moisture content, temperature and inoculation. Beitr Trop Landwirtsch Veterinarmed 14: 179–185
Fox RL, Atelsalp HM, Kampbell DH and Rhoades HF (1964a) Factors influencing the availability of sulfur fertilizers to alfalfa and corn. Proc Soil Sci Soc Am 28: 406–408
Fox RL, Flowerday AD, Hosterman FW, Rhoades HF and Olson RA (1964b) Sulfur fertilizers for alfalfa production in Nebraska. Nebraska Agric Exp Sta Bull No 214
Hutchinson KJ and Roper MM (1985) The importance of plant and animal residues in the nutrient economies of pasture and cropping systems. In: R.A. Leng et al., (eds). Reviews in Rural Science, 6, 207–218. Biotechnology and recombinant DNA technology in the animal production industries. Armidale: University of New England
Jones MN and Ruckman JE (1969) Effect of particle size on long-term availability of sulfur on annual-type grasslands. Agron J 61: 936–939
Keimnec G, Jackson TLK and Mosher W (1981) Fertilizing subclover with elemental sulfur. Sulfur in Agric 5: 12–16
Kittims HA and Attoe OJ (1965) Availability of phosphorus in rock phosphate-sulfur fusions. Agron J 57: 331–334
Li P and Caldwell AC (1966) The oxidation of elemental sulfur in soil. Proc Soil Sci Am 30: 370–372
McCaskill MR (1984) The residual effects of elemental sulfur as a pasture fertilizer. M Ru Sci Thesis. Armidale: University of New England
McCaskill MR and Blair GJ (1987) Particle size and soil texture effects on elemental sulfur oxidation. Agron J 79(6): 1079–1083
McCaskill MR and Blair GJ (1988) Development of a simulation model of sulfur cycling in grazed pastures. Biogeochem 5: 165–181
Moser US and Olson RV (1953) Sulfur oxidation in four soils as influenced by soil moisture tension and sulfur bacteria. Soil Sci 76: 251–256
Shedley CD (1982) An evaluation of elemental sulfur as a pasture fertilizer. PhD Thesis. Armidale: University of New England
Spencer K (1968) Availability to clover of sulfur as gypsum and brimstone of different particle sizes. Fld Stn Rec, Aust. CSIRO Div. Plant Industry 7: 1–12
Weir RG, Barkus B and Atkinson WT (1963) The effect of particle size on the availability of brimstone sulfur to white clover. Aust J Exp Agric Anim Husb 3: 314–318
Williams CH (1969) Moisture uptake by surface-applied superphosphate and movement of the phosphate and sulfate into the soil. Aust J Soil Res 7: 307–316
Williams CH (1971a) Reaction of surface-applied superphosphate with soil. 1. The fertilizer solution and its initial reaction with soil. Aust J Soil Res 9: 83–94
Williams CH (1971b) Reaction of surface-applied superphosphate with soil. 2. Movement of the phosphorus and sulfur into the soil. Aust J Soil Res 9: 95–106
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McCaskill, M.R., Blair, G.J. A model for the release of sulfur from elemental S and superphosphate. Fertilizer Research 19, 77–84 (1989). https://doi.org/10.1007/BF01054678
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01054678