Abstract
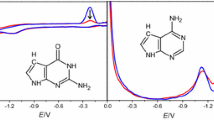
The adsorption and the reduction of native and denatured DNA has been studied at the hanging mercury drop electrode (HMDE) by triangular sweep polarography. It has been established from the dependence of the reduction peak of denatured DNA (Fig. 2) on the waiting time at the starting potential E8 of the sweep (Fig. 3) where the adsorption takes place and on pH (Fig. 4) that a protonated form of denatured DNA is reduced from the adsorbed state in a totally irreversible electrode process at a pH lower than 7.5. From the logarithmic analysis of the beginning part of the reduction peak the charge transfer rate constant and the charge transfer coefficient were determined (Fig. 5).
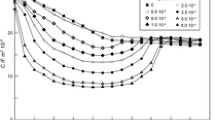
The reduction products are interconnected to a high molecular network strongly adhering to the surface of the electrode and blocking it for a repeated reduction on the same drop. In neutral media the protonation of DNA becomes rate-determining. The adsorption is diffusion controlled and from the time dependence of the coverage the diffusion coefficient of DNA could be estimated (Fig. 6) to 5.10−7 cm2 s−1 in 1 M KCl at 25 ‡C and at pH 6.7 for denatured DNA of the mean molecular weight 106 g. The protonated molecules of DNA are adsorbed via strong induced dipole interactions of theπ-electron system in the heteroaromatic ring of the bases with electrons of the metal while the other parts of the DNA molecule including the solvated sugar and phosphate groups are orientated to the solution.
From the time integral of the reduction peak (Fig. 6) the maximal surface concentration of the electrochemically active monomeric units in DNA was estimated to 1.4·10−10 mol cm−2 with an average area per mononucleotide of about 60 å2. Both values vary only within 10% in the temperature range 5 to 25 ‡C, in the concentration range of DNA 10 to 360 Μg/ml and for starting potentials Es −0.2 to −1.3 V vs. SCE.
Native DNA is also adsorbed at the electrode and reduced in acid solutions in the same irreversible electrode reaction as denatured DNA. However, before a partial opening of the helix is induced by the strong electric field at the interface leading with respect to helix-coil transition to an effect equivalent to partial melting which has to precede the electron transfer step. The extent of unscreened reducible groups and their orientation with the electrochemically reactive sites towards the electrode depends on pH (Fig. 8), on electrical parameters of the interface as the starting potential Es (Fig. 7) and on the portion of the DNA molecule which comes into the region where the electric field is acting. The maximal extents of unscreened reducible groups are 85% for native DNA of the mean molecular weight of 2.5·106 g and 46% for native DNA of the mean molecular weight 107 g (Fig. 6). The results have with respect to the physicochemical properties of DNA general significance also for the behaviour of nucleic acids at charged biological interfaces as for instance the membrane of living cells.
Similar content being viewed by others
References
Brabec, V., Paleček, E.: The influence of salts and pH on polarographic currents produced by denatured DNA. Biophysik6, 290–300 (1970)
Davidson, J. N.: The biochemistry of the nucleic acids, p. 57. London: Methuen 1969
Heyrovský, J., Kůta, J.: Principles of polarography, p. 205. New York: Interscience 1966
Hill, T. L.: Some possible biological effects of an electric field acting on nucleic acids or proteins. J. Am. Chem. Soc.80, 2142–2147 (1958)
Janik, B., Elving, P. J.: Polarographic behaviour of nucleosides and nucleotides of purines, pyrimidines and flavins. Chem. Rev.68, 295–319 (1968)
Nürnberg, H. W., Wolff, G.: Influences on homogeneous reactions in the diffuse double layer. J. Electroanal. Chem.21, 99–122 (1969)
Paleček, E., Vetterl, V.: Polarographic reducibility of denatured DNA. Biopolymers6, 917–928 (1968)
Phillips, S. L.: Diffusion-limited adsorption at spherical electrodes. J. Electroanal. Chem.12, 294–299 (1966)
Studier, F. W.: Sedimentation studies of the size and shape of DNA. J. Mol. Biol.11, 373–390 (1965)
Fasman, D., Timasheff, S. N.: Fine structure of proteins and nucleic acids. Chap. 2: Ts'o, P.O.P.: Monomeric units of nucleic acid-bases, nucleosides and nucleotides. New York: M. Dekker 1970
Valenta, P., Grahmann, P.: Electrochemical behaviour of natural and biosynthetic polynucleotides at the mercury electrode. I. The adsorption of denatured deoxyribonucleic acid at the hanging mercury drop electrode. J. Electroanal. Chem.49, 41–53 (1974)
Valenta, P., Nürnberg, H. W.: II. Reduction of denatured DNA at stationary and dropping mercury electrodes. J. Electroanal. Chem.49, 55–75 (1974)
Valenta, P., Nürnberg, H. W., Klahre, P.: III. A comparative study on the electrochemical properties of native and denatured DNA. Bioelectrochemistry and Bioenergetics (in press)
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. L. Holleck on his 70th birthday.
Rights and permissions
About this article
Cite this article
Valenta, P., Nürnberg, H.W. The electrochemical behaviour of DNA at electrically charged interfaces. Biophys. Struct. Mechanism 1, 17–26 (1974). https://doi.org/10.1007/BF01022557
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01022557