Abstract
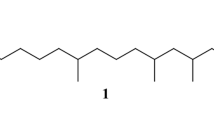
A simple three-step synthesis is described for 6-acetoxy-5-hexadecanolide, the oviposition pheromone of the mosquitoCulex quinquefasciatus Say and others in that genus. An aldol condensation between 1-trimethylsilyloxycyclopent-1-ene and undecanal, followed by Baeyer-Villiger ring expansion and acetylation, gave the required compound as a 1∶1 mixture of diastereoisomers in high overall yield (>80%). This synthetic approach is readily adapted for synthesis of analogs. The heptadecafluoro compound, in which then-octyl group is replaced by perfluorooctyl, retained high biological activity.
Similar content being viewed by others
References
Banks, R.E., andTatlow, J.C. 1986. A guide to modem organofluorine chemistry,in R.E. Banks, D.W.A. Sharp, and J.C. Tatlow (eds.). Fluorine. The First Hundred Years (1886–1986). Elsevier Sequoia, Lausanne, pp. 227–284.
Barua, N.C., andSchmidt, R.R. 1986. Stereoselective synthesis of the major component of a mosquito oviposition attractant pheromone from a β-lithiopropionate equivalent.Tetrahedron 42:4471–4474.
Briggs, G.G., Cayley, G.R., Dawson, G.W., Griffiths, D.C., Macaulay, E.D.M., Pickett, J.A., Pile, M.M., Wadhams, L.J., andWoodcock, C.M. 1986. Some fluorine-containing pheromone analogues.Pestic. Sci. 17:441–448.
Bruno, D.W., andLaurence, B.R. 1979. The influence of the apical droplet ofCulex egg rafts on oviposition ofCulex pipiens fatigans (Diptera: Culicidae).J. Med. Entomol. 16:300–305.
Fuganti, C.,Grasselli, P., andServi, S. 1982. Synthesis of the two enantiomeric forms oferythro-6-acetoxy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone.J. Chem. Soc. Chem. Commun. 1285–1286.
Hwang, Y.-S., Mulla, M.S., Chaney, J.D., Lin, G.-Q., andXu, H.-J. 1987. Attractancy and species specificity of 6-acetoxy-5-hexadecanolide, a mosquito oviposition attractant pheromoneJ. Chem. Ecol. 13:245–252.
Jefford, C.W., Jaggi, D., andBoukouvalas, J. 1986. A short, stereodivergent synthesis of the racemicerythro andthreo diastereomers of 6-acetoxy-5-hexadecanolide, a mosquito oviposition attractant pheromone.Tetrahedron Lett. 27:4011–4014.
Ko, K.-Y., andEliel, E.L. 1986. Asymmetric synthesis of (5R, 6S)-6-acetoxy-5-hexadecanolide, the major component of the oviposition attractant pheromone of the mosquitoCulex pipiens fatigans, and two of its stereoisomers.J. Org. Chem. 51:5353–5362.
Laurence, B.R., andPickett, J.A. 1982.erythro-6-Acetoxy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone.J. Chem. Soc. Chem. Commun. 59–60.
Laurence, B.R., andPickett, J.A. 1985. An oviposition attractant pheromone inCulex quinquefasciatus Say (Diptera: Culicidae).Bull. Entomol. Res. 75:283–290.
Laurence, B.R., Mori, K., Otsuka, T., Pickett, J.A., andWadhams, L.J. 1985. Absolute configuration of the mosquito oviposition attractant pheromone, 6-acetoxy-5-hexadecanolide.J. Chem. Ecol. 11:643–648.
Lin, G.-Q., Xu, H.-J., Wu, B.-C., Guo, G.-Z., andZhou, W.-S. 1985. Studies on the identification and synthesis of insect pheromones. XXI. Stereoselective synthesis of all the possible optical isomers of the mosquito oviposition attractant pheromone.Tetrahedron Lett. 26:1233–1236.
Machiya, K., Ichimoto, I., Kirihata, M., andUeda, H. 1985. A convenient synthesis of four stereoisomers of 6-acetoxy-5-hexadecanolide, the major component of the mosquito oviposition attractant pheromone.Agric. Biol. Chem. 49:643–649.
Mori, K., andOtsuka, T. 1983. Synthesis of both the enantiomers oferythro-6-acetoxy-5-hexadecanolide.Tetrahedron 39:3267–3269.
Mukaiyama, T., Banno, K., andNarasaka, K. 1974. New cross-aldol reactions. Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride.J. Am. Chem. Soc. 96:7503–7509.
Ochiai, M.,Ukita, T.,Nagao, Y., andFujita, E. 1985. Stereochemistry of an oxidative 1,4-fragmentation of γ-stannyl alcohols with a hypervalent organoiodine compound and the synthesis oferythro-6-acetoxyhexadecan-5-olide.J. Chem. Soc. Chem. Commun. 637–638.
Otieno, W.A., Onyango, T.O., Pile, M.M., Laurence, B.R., Dawson, G.W., Wadhams, L.J., andPickett, J.A. 1988. A field trial of the synthetic oviposition pheromone withCulex quinquefasciatus Say (Diptera: Culicidae) in Kenya.Bull. Entomol. Res. 78:463–470.
Prestwich, G. D., Wei-Chuan, Sun, Mayer, M. S., andDickens, J. C. 1990. Perfluorinated mothpheromones: Synthesis and electrophysiological activity.J. Chem. Ecol. 16:1761–1778.
Sato, T.,Watanabe, M.,Honda, N., andFujisawa, T. 1984. Synthesis of both enantiomers oferythro-6-acetoxy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone.Chem. Lett. 1175–1176.
Wang, Z-M., Qian, X-H., andZhou, W-S. 1990. Stereoselective synthesis of (−)-(5R,6S)-6-acetoxy-5-hexadecanolide, the mosquito oviposition attractant pheromone.Tetrahedron 46:1191–1198.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dawson, G.W., Mudd, A., Pickett, J.A. et al. Convenient synthesis of mosquito oviposition pheromone and a highly fluorinated analog retaining biological activity. J Chem Ecol 16, 1779–1789 (1990). https://doi.org/10.1007/BF01020494
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01020494