Abstract
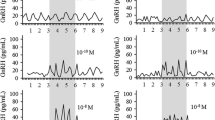
Using an in vitro perifusion system, the present study investigated the possibility that alterations in catecholamine and luteinizing hormone-releasing hormone (LHRH) secretion from the male rat mediobasal hypothalamus are present during the period of middle-age. The results indicate that, while tissue concentrations and baseline secretion of norepinephrine, dopamine and LHRH were similar between age groups, the patterns of dopamine and LHRH release in response to a series of depolarizing stimuli was different in the older animals. After all challenges, dopamine concentrations in the perifusate declined much more sharply for the middle-aged group, a finding that may be associated with a decrease with age in the pool of transmitter available for ready release. Also, tissue fragments from young adult rats were able to maintain the release of LHRH to a greater extent than tissue from the middle-aged animals, but only for the initial challenge period. The typical episodic pattern of LHRH release appeared to be disrupted in the older group following a second stimulus. It is possible that these age-related changes are early components of a dysruption in the hypothalamic mechanisms governing gonadotropin secretion.
Similar content being viewed by others
References
Becker, J., and Ramirez, V. D. 1980. Dynamics of endogenous catecholamine release from brain fragments of male and female rats. Neuroendocrinology 31:18–25.
Brabant, G., Ray, A. K., Wagner, T. O. F., Krech, R., and von zur Mühlen, A. 1985. Peripubertal variation of GnRH-release from in vitro superfused hypothalami of male rats. Neuroendocrinol. Lett. 7:197–202.
Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein dye binding. Anal. Biochem. 72:248–254.
Bruni, J. F., Huang, H. H., Marshall, S., and Meites, J. 1977. Effects of single and multiple injections of synthetic GnRH on serum LH, FSH and testosterone in young and old male rats. Biol. Reprod. 17:309–312.
Charli, J. L., Rotsztejn, W. H., Pattou, E., and Kordon, C. 1978. Effect of neurotransmitters on in vitro release of luteinizing hormone-releasing hormone from the mediobasal hypothalamus of male rats. Neurosci. Lett. 10:159–163.
Colombani-Vidal, M., and Barnea, A. 1985. Effects of castration and maturational age of male rats on the process of copper-stimulated release of luteinizing hormone releasing hormone from median eminence explants: Evidence that androgens increase the affinity of the copper-interactive sites for copper. Neuroendocrinology 41:454–461.
Cooper, R. L., and Walker, R. F. 1983. Radioenzymatic assays for catecholamines and serotonin in brain and blood. Pages 483–493,in Conn, P. M. (ed.) Methods in Enzymology, Vol. 103, Neuroendocrine Peptides. Acad. Press, N.Y.
Drouva, S. V., Epelbaum, J., Hery, M., Tapia-Arancibia, L., Laplante, E., and Kordon, C. 1981. Ionic channels involved in the LHRH and SRIF release from rat mediobasal hypothalamus. Neuroendocrinology 32:155–162.
Dyer, R. G., Mansfield, S., and Yates, J. O. 1980. Discharge of gonadotrophin-releasing hormone from the mediobasal part of the hypothalamus: Effect of stimulation frequency and gonadal steroids. Exp. Brain Res. 39:453–460.
Estes, K. S., and Simpkins, J. W. 1980. Age-related alterations in catecholamine concentrations in discrete preoptic area and hypothalamic regions in the male rat. Brain Res. 194:556–560.
Estes, K. S., and Simpkins, J. W. 1982. Resumption of pulsatile luteinizing hormone release after α-adrenergic stimulation in aging constant estrous rats. Endocrinology 111:1778–1784.
Glowinski, J. 1975. Properties and functions of intraneuronal monoamine compartments in central aminergic neurons. Pages 139–167,in Iverson, L. L., Iverson, S. D., and Snyder, S. H. (eds.), Handbook of Pharmacology, Plenum Press, N.Y.
Goldman, J. M., Cooper, R. L., Rehnberg, G. L., Hein, J. F., McElroy, W. K., and Gray, L. E., Jr. 1986. Effects of low, subchronic doses of methoxychlor upon the rat hypothalamic-pituitary reproductive axis. Toxicol. Appl. Pharmacol. 86:474–483.
Goldman, J. M., Walker, R. F., and Cooper, R. L. 1985. Aging in the rat hypothalamic-pituitary-ovarian axis: The involvement of biogenic amines in the loss of reproductive cyclicity. Pages 127–152,in Parvez, H., Parvez, S., and Gupta, D. (eds.), Neuroendocrinology of Hormone-Transmitter Interaction, Progress in Neuroendocrinology, Vol. 1. V.N.U. Int. Sci. Press, Utrecht.
Gray, G. D., Smith, E. R., and Davidson, J. M. 1980. Gonadotropin regulation in middle-aged male rats. Endocrinology 107:2021–2026.
Greenwood, F. C., Hunter, W. M., and Glover, J. S. 1963. The preparation of131labelled human growth hormone of high specific activity. Biochem. J. 89:114–123.
Heber, D., Odell, W. D., Schedewie, H., and Wolfson, A. R. 1978. Improved iodination of peptides for radioimmunoassay and membrane radioreceptor assay. Clin. Chem. 24:796–799.
Hoffman, G. E., and Sladek, J. R., Jr. 1980. Age-related changes in dopamine, LHRH and somatostatin in the rathypothalamus. Neurobiol. Aging 1:27–37.
Jackson, A. J., and Franklin, R. B. 1984. Liposome-entrapped diethylstilbestrol effect on prostate gland and plasma testosterone levels in rats. Endocrinology 115:538–543.
Jarjour, L. T., Handelsman, D. J., and Swerdloff, R. S. 1986. Effects of aging on the in vitro release of gonadotropin-releasing hormone, Endocrinology 119:1113–1117.
Jennes, L., and Stumpf, W. E. 1983. Preparation and use of specific antibodies for immunohistochemistry of neuropeptides. Pages 448–459,in Conn, P. M. (ed.), Methods in Enzymology, Vol. 103, Neuroendocrine Peptides, Academic Press, N.Y.
Kalra, P. S., and Kalra, S. P. 1982. Discriminative effects of testosterone on hypothalamic luteinizing hormone-releasing hormone levels and luteinizing hormone secretion in castrated male rats: Analyses of dose and duration characteristics. Endocrinology 111:24–29.
de Langen, C. D. J., Stoof, J. C., and Mulder, A. H. 1979. Studies on the nature of the releasable pool of dopamine in synaptosomes from rat corpus striatum: Depolarization-induced release of3H-dopamine from superfused synaptosomes labelled under various conditions. N.-S. Arch. Pharmacol. 308:41–49.
Levy, W. B., Haycock, J. W., and Cotman, C. W. 1976. Stimulation-dependent depression of readily releasable neurotransmitter pools in brain. Brain Res. 115:243–256.
Miller, A. E., and Riegle, G. D. 1975. Aging effects on hypothalamic catecholamine and testosterone secretion in the aging male rat. Fed. Proc. 34:303.
Miller, A. E., and Riegle, G. D. 1978. Serum LH levels following multiple LHRH injections in aging rats. Proc. Soc. Exp. Biol. Med. 157:494–499.
Miller, A. E., and Riegle, G. D. 1978. Serum testosterone and testicular response to HCG in young and aged male rats. J. Gerontol. 33:197–203.
Miller, A. E., and Riegle, G. D. 1982. Temporal patterns of serum luteinizing hormone and testosterone and endocrine response to luteinizing hormone releasing hormone in aging male rats. J. Gerontol. 37:522–528.
Miller, A. E., Shaar, C. J., and Riegle, G. D. 1976. Aging effect on hypothalamic dopamine and norepinephrine content in the male rat. Exp. Aging Res. 2:475–480.
Miyake, A., Tasaka, K., Sakumoto, T., Kawamura, Y., Aono, T., and Kurachi, K. 1983. Norepinephrine induces releases of both LH-RH from the hypothalamus and LH from the rat pituitary in vitro. Endocrinol. Japon. 30:509–512.
Nett, T. M., and Niswender, G. D. 1979. Gonadotropin-releasing hormone. Pages 57–75,in Jaffe, B. M., and Behrman, H. R. (eds.), Methods of Hormone Radioimmunoassay, 2nd ed., Academic Press, N.Y.
Ojeda, S. R., Harms, P. G., and McCann, S. M. 1974. Effect of blockade of dopaminergic receptors on prolactin and LH release: Median eminence and pituitary sites of action. Endocrinology 94:1650–1657.
Peterson, C., and Gibson, G. E. 1983. Amelioration of agerelated neurochemical and behavioral deficits by 3,4-diaminopyridine. Neurobiol. Aging 4:25–30.
Peterson, C., Nicholls, D. G., and Gibson, G. E. 1985. Subsynaptosomal distribution of calcium during aging and 3,4-diaminopyridine treatment. Neurobiol. Aging 6:297–304.
Rance, N., and Barraclough, C. A. 1981. Effects of phenobarbital on hypothalamic LHRH and catecholamine turnover rates in proestrous rats. Proc. Soc. Exp. Biol. Med. 166:425–431.
Rotsztejn, W. H., Charli, J. L., Pattou, E., Epelbaum, J., and Kordon, C. 1976. In vitro release of luteinizing hormonereleasing hormone (LHRH) from rat mediobasal hypothalamus: Effects of potassium, calcium and dopamine. Endocrinology 99:1663–1666.
Rotsztejn, W. H., Charli, J. L., Pattou, E., and Kordon, C. 1977. Stimulation by dopamine of luteinizing hormone-releasing hormone (LHRH) release from the mediobasal hypothalamus in male rats. Endocrinology 101:1475–1483.
Sartin, J. L., and Lamperti, A. A. 1985. Neuron numbers in hypothalamic nuclei of young, middle-aged and aged male rats. Experientia 41:109–111.
Schoffelmeer, A. N. M., and Mulder, A. H. 1983. [3H]Noradrenaline release from brain slices induced by an increase in the intracellular sodium concentration: Role of intracellular calcium stores. J. Neurochem. 40:615–621.
Schoffelmeer, A. N. M., Wemer, J., and Mulder, A. H. 1981. Comparison between electrically evoked and potassium-induced3H-noradrenaline release from rat neocortex slices: Role of calcium ions and transmitter pools. Neurochem. Int. 3:129–136.
Shaar, C. J., Euker, J. S., Riegle, G. D., and Meites, J. 1975. Effects of castration and gonadal steroids on serum LH and prolactin in old and young rats. J. Endocrinol. 66:45–51.
Simpkins, J. W., Estes, K. S., Kalra, P. S., and Kalra, S. P. 1979. Age-related alterations in catecholamine and LHRH concentration in brain nuclei of the male rat. Page 31in Korenman, S. G. (ed.), Endocrine Aspects of Aging, Raven Press, N.Y.
Sonntag, W. E., Forman, L. J., Fiori, J. M., Hylka, V. W., and Meites, J. 1984. Decreased ability of old make rats to secrete luteinizing hormone (LH) is not due to alterations in pituitary LH-releasing hormone receptors. Endocrinology 114:1657–1664.
Steger, R. W., and Huang, H. H. 1983. The reproductive decline in male rats. Pages 123–142,in Meites, J. (ed.), Neuroendocrinology of Aging, Plenum Press, N.Y.
Steger, R. W., De Paolo, L. V., and Shepherd, A. M. 1985. Effects of advancing age on hypothalamic neurotransmitter content and on basal and norepinephrine-stimulated LHRH release. Neurobiol. Aging 6:113–116.
Steiner, R. A., Bremner, W. J., Clifton, D. K., and Dorsa, D. M. 1984. Reduced pulsatile luteinizing hormone and testosterone secretion with aging in the male rat. Biol. Reprod. 31:251–258.
Vijayan, E., and McCann, S. M. 1978. Re-evaluation of role of catecholamines in control of gonadotropin and prolactin release. Neuroendocrinology 25:150–165.
Wilkes, M. M., Lu, K. H., Hopper, B. R., and Yen, S. S. C. 1979. Altered neuroendocrine status of middle-aged rats prior to the onset of senescent anovulation. Neuroendocrinology 29:255–261.
Wise, P. M. 1982. Norepinephrine and dopamine activity in microdissected brain areas of the middle-aged and young rat on proestrus. Biol. Reprod. 27:562–574.
Zamora, A. J., and Ramirez, V. D. 1983. Structural changes in nerve endings of rat median eminence superfused with media rich in potassium ions. Neuroscience 10:463–473.
Author information
Authors and Affiliations
Additional information
The research described in this article has been reviewed by the Health Effects Research Laboratory, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade name or commercial products constitute endorsement or recommendation for use.
Rights and permissions
About this article
Cite this article
Goldman, J.M., Cooper, R.L., Rehnberg, G.L. et al. Age-related alterations in the stimulated release in vitro of catecholamines and luteinizing hormone-releasing hormone from the male rat hypothalamus. Neurochem Res 12, 651–657 (1987). https://doi.org/10.1007/BF00971015
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00971015