Abstract
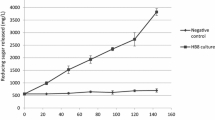
Five psychrophilic Antarctic bacteria have been selected for their capacity to secrete exoenzymes into culture medium. These strains are able to grow from 0 to about 25° C. However, production of lipase fromMoraxella, α-amylase fromAlteromonas haloplanctis, β-lactamase fromPsychrobacter immobilis and protease fromBacillus is maximal at temperatures close to that of their environment (—2 to 4° C) and is strongly inhibited at higher temperatures. This thermal effect involves alterations in the secretory pathway in the upper range of temperatures, losses due to the enzyme thermal lability and in some cases to reduction in cell development. The apparent optimal activity temperature of these enzymes is between 30 and 40° C, i.e. about 20° C lower than that of their mesophilic counterparts.
Similar content being viewed by others
References
Dambmann C, Aunstrup K (1981) The variety of serine proteases and their industrial significance. In: Turk V, Vitale L (eds) Proteinases and their inhibitors. Structure, function and applied aspects. Pergamon Press, Oxford, pp 231–244
Feller G, Thiry M, Arpigny JL, Gerday C (1991) Cloning and expression inEscherichia coli of three lipase-encoding genes from the psychrotrophic Antarctic strainMoraxella TA144. Gene 102:111–115
Gounot A-M (1991) Bacterial life at low temperature: physiological aspects and biotechnological implications. J Appl Bacteriol 71:386–397
Hägele EO, Kratzer M, Schaich E, Rauscher E (1989) Mechanism of action of human pancreatic and salivary α-amylase on 4,6-ethylidene-α-4-nitrophenyl maltoheptaoside substrate. Clin Chem 35:188–189
Hazen GG, Hause JA, Hubicki JA (1965) An automated system for the quantitative determination of proteolytic enzymes using azocasein. Ann N Y Acad Sci 130:761–768
Khalid A, Rahim A, Lee BH (1991) Production and characterization of β-galactosidase from psychrotrophicBacillus subtilis KL88. Biotechnol Appl Biochem 13:246–256
Kobori H, Sullivan CW, Shizuya H (1984) Heat-labile phosphatase from Antarctic bacteria: rapid 5′ end-labeling of nucleic acids. Proc Natl Acad Sci USA 81:6691–6695
Kolenc RJ, Inniss WE, Glick BR, Robinson CW, Mayfield CI (1988) Transfer and expression of mesophilic plasmid-mediated degradative capacity in a psychrotrophic bacterium. Appl Environ Microbiol 54:638–641
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167
Margesin R, Schinner F (1991) Characterization of a metalloprotease from psychrophilicXanthomonas maltophilia. FEMS Microbiol Lett 79:257–262
Russell NJ (1990) Cold adaptation of micro-organisms. Phil Trans R Soc Lond B326:595–611
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feller, G., Narinx, E., Arpigny, J.L. et al. Temperature dependence of growth, enzyme secretion and activity of psychrophilic Antarctic bacteria. Appl Microbiol Biotechnol 41, 477–479 (1994). https://doi.org/10.1007/BF00939039
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00939039