Abstract
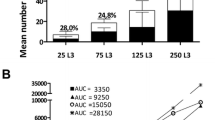
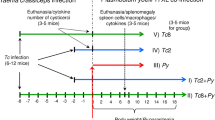
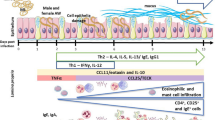
The time course of intestinal infection of golden hamsters (Mesocricetus auratus) withTaenia crassiceps was monitored every 2 days up to day 26 postinfection (p.i.). The isolate used was originally obtained fromClethrionomys rutilus on St. Lawrence Island, Bering Sea (USA), and shows a high level of enteral establishment without parenteral infection. Following oral administration of various numbers (3, 15, 30, or 60) of metacestodes, proportional numbers of cestodes attached their scolices to the mucosa of the middle (onethird) segment of the small intestine. Except for animals given 3 metacestodes, dislocation of cestodes to the posterior parts of the intestine was noted on days 10–14 p.i. and began faster in animals that had received larger numbers of metacestodes. Concurrent with dislocation or elimination of cestodes, there existed distinct increases in intestinal eosinophil peroxidase and myeloperoxidase activities. The number of mast cells in the lamina propria peaked on day 16 p.i., and specific serum IgG began to increase within 1 week p.i. The level of all these changes was dose-dependent. In prednisolone-treated animals that received 60 metacestodes, dislocation of cestodes was not evident, and mucosal inflammatory changes were suppressed to a considerable extent. The findings of this study are discussed as a model for the study of canine taeniasis.
Similar content being viewed by others
References
Andreassen J, Hindsbe O, Vienberg S (1982) Responsiveness of congenitally thymus deficient nude mice to the intestinal cestode,Hymenolepis diminuta. Int J Parasitol 12:215–219
Befus AD, Threadgold LT (1975) Possible immunological damage to the tegument ofHymenolepis diminuta in mice and rats. Parasitology 71:525–534
Chappell LH, Pike AW (1976) Loss ofHymenolepis diminuta from the rat. Int J Parasitol 6:333–339
Craig PS, Rickard MD (1982) Evaluation of ‘crude’ antigen prepared fromTaenia saginata for the serological diagnosis ofT. saginata cysticercosis in cattle using the enzyme-linked immunosorbent assay (ELISA). Z Parasitenkd 61:287–297
Cramer R, Soranzo MR, Dri P, Menegazzi R, Pitotti A, Zabucchi G, Patriarca P (1984) A simple reliable assay for myeloperoxidase activity in mixed neutrophil-eosinopil cell suspensions; application to detection of myeloperoxidase deficiency. J Immunol Methods 70:119–125
Heath DD, Lawrence SB, Glennie A, Twaalfhoven H (1985) The use of excretory and secretory antigens of the scolex ofTaenia ovis for the serodiagnosis of infection in dogs. J Parasitol 71:192–199
Hopkins CA (1980) Immunity andHymenolepis diminuta. In: Arai HP (ed) Biology of the tapewormHymenolepis diminuta. Academic Press, New York, pp 551–614
Hopkins CA, Subramanian G, Stallard H (1972) The development ofHymenolepis diminuta in primary and secondary infections in mice. Parasitology 64:401–412
Ito A, Smyth JD (1987) Adult cestodes; immunology of the lumen-dwelling cestode infections. In: Soulsby EJL (ed) Immune responses in parasitic infections: immunology, immunopathology, and immunoprophylaxis; vol 2. Trematodes and cestodes. CRC Press, Boca Raton, Florida, pp 115–163
Jenkins DJ, Rickard MD (1986) Specific antibody responses in dogs experimentally infected withEchinococcus granulosus. Am J Trop Med Hyg 35:345–349
Kitaoka M, Oku Y, Okamoto M, Kamiya M (1990) Development and sexual maturation ofTaenia crassiceps (Cestoda) in the golden hamster. J Parasitol 76:399–402
Kinder A, Carter SD, Allan J, Marshall-Clarke S, Craig PS (1992) Salivary and serum antibodies in experimental canine taeniasis. Vet Parasitol 41:321–327
Kroeze WK, Freeman RS (1982)Taenia crassiceps: fate of cysticerci following ingestion by the mouse. Exp Parasitol 54:425–431
Lillie RD (1965) Histopathologic technique and practical histochemistry, McGraw Hill Co.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
McCaigue MD, Halton DW (1987) Immunological damage toHymenolepis diminuta following a challenge infection in C57 mice. Int J Parasitol 17:795–803
Moqbel R (1986) Helminth-induced intestinal inflammation. Trans R Soc Trop Med Hyg 80:719–727
Moqbel R, MacDonald AJ (1990) Immunological and inflammatory responses in the small intestine associated with helminthic infections. In: Behnke JM (ed) Parasites: immunity and pathology. Taylor & Francis, London, pp 249–282
Rothwell TLW (1989) Immune expulsion of parasitic nematodes from the alimentary tract. Int J Parasitol 19:139–168
Sato H, Oku Y, Rausch RL, Kamiya M (1993) Establishment and survival of the strobilar stage ofTaenia crassiceps in hamsters, gerbils and mice, with reference to different helminth isolates. Parasitol Res 79:619–623
Author information
Authors and Affiliations
Additional information
This study was supported by grants 01790490, 02044083 and 03044016 from the Ministry of Education, Science and Culture, Japan
Rights and permissions
About this article
Cite this article
Sato, H., Kamiya, H., Oku, Y. et al. Infection course of the strobilar stage ofTaenia crassiceps in golden hamsters, with reference to host responses. Parasitol Res 80, 99–103 (1994). https://doi.org/10.1007/BF00933774
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00933774