Abstract
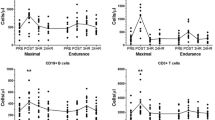
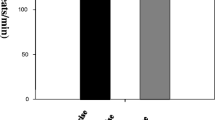
The effect of acute exercise on natural killer (NK) activity and on the distribution of phenotypic characteristics of peripheral blood lymphocytes was examined. Trained and sedentary individuals underwent a standard progressive exercise test on a cycle ergometer using an incremental work load of 15 W (90 kpm), increased every minute. Each subject was encouraged to exercise to exhaustion, and total ventilation and mixed expired O2 and CO2 were measured every 30 sec. All subjects reached the “anaerobic” threshold as judged by the deflection of ventilation at a work load nearVO2max. NK activity against K562 reached maximum levels immediately after exercise, dropped to a low point 120 min later, then slowly came back to preexercise levels within 20 hr. No significant differences were observed between the trained and the sedentary groups. Furthermore, immediately after exercise the proportion of OKT-3+ and OKT-4+ cells was reduced by 29.8±3.6 and 33.6±5.4%, respectively; the percentage Leu-7+ and Leu-11a+ cells was increased by 53.9±1.7 and 57.3±2.9%, respectively. The percentage OKT-8+ cells was not significantly altered. When the percentage binding of effector to target cells was examined, it was highest at 0 min post-exercise (19±6.2%) and lowest at 120 min postexercise (7±3.9%), but the absolute number of NK cells remained unchanged. The source of serum used in the lytic assay had no effect on the NK activity, as fetal calf serum and autologous sera drawn at different time intervals during exercise gave similar results. Epinephrine suppressed and interferon enhanced the NK activity of all cells tested, which indicates that during and after exercise NK cells did not go through a refractory stage. Our results show that, contrary to other lymphocyte subpopulations, NK cells are resistant to the depletive and modulatory effects of acute exercise and the increase in NK activity is not due to an increase in the absolute number of NK cells.
Similar content being viewed by others
References
Sklar SL, Anisman H: Stress and coping factors influence tumor growth. Science 204:513, 1979
Sheffer AL, Auten KF: Exercise-induced anaphylaxis. J Allergy Clin Immunol 66:106, 1980
Aarstadt HJ, Gaudernack G, Seljelid R: Stress causes reduced natural killer activity in mice. Scand J Immunol 18:461, 1983
Borysenko M, Turesky S, Borysenko JZ, Quimby F, Benson H: Stress and dental caries in the rat. J Behav Med 3:233, 1980
Solomon GF: Stress and antibody response in rats. Int Arch Allergy Appl Immunol 35:97, 1969
Jemott JB, Borysenko M, Chapman R, Borysenko J, McClelland D, Myer D: Academic stress, power motivation, and decrease in secretion rate of salivary secretory immunoglobulin A. Lancet 1(8389):1400, 1983
Eskola J, Ruuskanen O, Soppi E, Viljanen MK, Jarvinen M, Toivonen H, Kouvalainen M: Effect of sport stress on lymphocyte transformation and antibody formation. Clin Exp Immunol 32:339, 1978
Ursin H: Personality, activation and somatic health, a new psychosomatic theory.In Coping and Health, S Levine, H Ursin (eds). 1980
Shavit Y, Lewis JW, Terman GW, Gale RP, Liebeskind JC: Opioids peptides mediate the suppressive effect of stress on natural killer cell activity. Science 23:188, 1984
Vischer TL: Effect of hydrocortisone on the reactivity of thymus and spleen cells of mice to in vitro stimulation. Immunology 23:777, 1972
Bliznakov EG: Serotonin and its precursors as modulators of the immunological responsiveness in mice. J Med 11:81, 1980
Johnson HM, Smith EM, Torres BA, Blalock JE: Regulation of the in vitro antibody response by neuroendocrine hormones. Proc Natl Acad Sci USA 79:4171, 1982
Cross RJ, Markesberry WR, Brooks WH, Roszman TL: Hypothalamic-immune interactions: Neuromodulation of natural killer activity by lesioning of the anterior hypothalamus. Immunology 51:399, 1984
Levi F, Canon C, Blum JP, Reinberg A, Mathe G: Large-amplitude circadian rhythm in helper/suppressor ratio of peripheral blood lymphocytes. Lancet 2(8347):462, 1983
Fischer DG, Hubbard WJ, Koren HS: Tumor cell killing by freshly isolated peripheral blood monocytes. Cell Immunol 58:426, 1981
Lozzio CB, Lozzio BB: Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45:321, 1975
Brahmi Z: Nature of natural killer cell hyporesponsiveness in the Chediak-Higashi syndrome. Hum Immunol 6:45, 1983
Blair BP, Staskawicz MO, Sam JS: Inhibitor cells in spleens of mice with low natural killer cell activity. JNCI 71:571, 1983
Brahmi Z, Lazarus KH, Hodes ME, Baehner RL: Immunologic studies of three family members with the immunodeficiency with hyper-IgM syndrome. J Clin Immunol 3:127, 1983
Crary B, Hauser SL, Borysenko M, Kutz I, Hoban C, Ault KA, Weiner HL, Benson H: Epinephrine-induced changes in the distribution of lymphocyte subsets in peripheral blood of humans. J Immunol 131:1178, 1981
Herberman RB, Ortaldo JB, Bonnard GD: Augmentation by inteferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature 277:221, 1979
Katz P, Zaytoun A, Fauci AS: Modulation of natural killer cell activity by cyclic nucleotides. J Immunol 129:287, 1982
Bray R, Abrams S, Brahmi Z: Studies on the mechanism of human natural killer cell-mediated cytolysis. Modulation by dexamethasone and arachidonic acid. Cell Immunol 78:100, 1983
Haynes BF, Katz P, Fauci AS: Mechanisms of corticosteroids action on lymphocyte subpopulation. Cell Immunol 44:169, 1979
Hoffman T, Hizata T, Bougnoux P, Fraser BA, Goldfarb R, Herberman RB, Axelrod J: Phospholipid methylation and phospholipase A2 activation in cytotoxicity by natural killer cells. Proc Natl Acad Sci USA 78:3839, 1981
Lanier L, Le AM, Phillips JH, Warner NL, Babcok GF: Subpopulations of human natural killer cells defined by the expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol 131:1789, 1983
Keller SE, Weiss JM, Scheifer SJ, Miller NE, Stein M: Stressed-induced suppression of immunity in adrenalectomized rats. Science 22:1301, 1983
Katz P, Zaytoun AM, Lee HJ: The effects of in vivo hydrocortisone on lymphocyte-mediated cytotoxicity. Arth Rheum 27:72, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brahmi, Z., Thomas, J.E., Park, M. et al. The effect of acute exercise on natural killer-cell activity of trained and sedentary human subjects. J Clin Immunol 5, 321–328 (1985). https://doi.org/10.1007/BF00918251
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00918251