Abstract
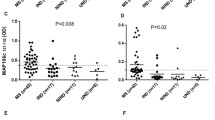
The role of human spumaretrovirus (HSRV) infections in the pathogenesis of multiple sclerosis (MS) was investigated with recombinant HSRV env-specific enzyme-linked immunosorbent assay. The presence of HSRV antibodies was determined in pairs of serum and cerebrospinal fluid (CSF) samples from 60 MS patients. In 7 of these patients serial serum and CSF samples were obtained in relation to the clinical activity of the disease during a period of 2 years. No increased antibody reactivity was demonstrable in the MS population compared with 14 aseptic meningitis patients, 50 blood donors and 16 healthy controls. Slightly elevated levels of antibodies were demonstrable in serum and/or CSF in 4 MS patients but also in 1 patient with aseptic meningitis, 1 blood donor and 1 child. No marked serum or CSF HSRV antibody fluctuation was observed in the MS patients followed longitudinally. Thus, this study does not support the involvement of HSRV in the pathogenesis of MS.
Similar content being viewed by others
References
Bartholomä A, Muranyi A, Flügel RM (1992) Bacterial expression of the capsid antigen domain and identification of native gag proteins in spumavirusinfected cells. Virus Res 23:27–38
Bothe K, Aguzzi A, Lassmann H, Rethwilm A, Horak I (1991) Progressive encephalopathy and myopathy in transgenic mice expressing human foamy virus genes. Science 253: 555–557
Brown P, Neomo G, Gajdusek DC (1978) Human foamy virus: further characterization, seroepidemiology, and relationship to chimpanzee foamy viruses. J Infect Dis 137:421–427
Cameron KR, Birchall SM, Moses MA (1978) Isolation of foamy virus from patient with dialysis encephalopathy. Lancet 11:796
Ehrlich GD, Glaser JB, Bryz-Gornia V, Maese J, Waldmann TA, Poiesz BJ, Greenberg SJ (1991) Multiple sclerosis, retroviruses, and PCR. Neurology 41:335–343
Flügel RM (1991) Spumaviruses: a group of complex retroviruses. J Acquir Immune Defic Syndr 4:739–750
Fugger L, Morling N, Ryder LP, Sandberg-Wollheim M, Svejgaard A (1990) Failure to demonstrate human T cell lymphotropic virus type I in multiple sclerosis patients. J Gen Virol 71: 1103–1107
Gajdusek DC, Rogers NG, Basnight M, Gibbs CJ Jr, Alpers M (1969) Transmission experiments with kuru in chimpanzees and the isolation of latent viruses from the explanted tissues of affected animals. Ann NY Acad Sci 162:529–550
Greenberg SJ, Ehrlich GD, Abbot MA, Hurwitz BJ, Waldmann TA, Poiesz BJ (1989) Detection of sequences homologous to human retroviral DNA in multiple sclerosis by gene amplification. Proc Natl Acad USA 86:2878–2882
Haahr S, Sommerlund M, MollerLarsen A, Nielsen R, Hansen HJ (1991) Just another dubious virus in cells from a patient with multiple sclerosis? Lancet 337:863–864
Hauser SL, Aubert C, Burks JS, Kerr C, Lyon-Caen O, The G de, Brahic M (1986) Analysis of human T-lymphotropic virus sequences in multiple sclerosis tissue. Nature 322:176–177
Karpas A, Malik K, Lida J (1987) Studies of human retroviruses in relation to adult T-cell leukaemia, acquired immune deficiency syndrome, and multiple sclerosis. Arch Virol 95: 237–249
Koprowski H, DeFreitas EC, Harper ME, Sandberg-Wollheim M, Sheremata WA, Robert-Guroff M, Saxinger CW, Feinberg MB, Wong-Staal F, Gallo RC (1985) Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature 318:154–160
Kurtzke JF (1984) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Lycke J, Anderson O, Svennerholm B, Ben-Menachem E, Horal P, Vahlne A (1992) Use of immunoreactive synthetic HTLV-1 peptides in search for antibody reactivity in multiple sclerosis. Acta Neurol Scand 85:44–45
Madden DL, Mundon FK, Tzan NR, Fuccillo DA, Dalakas MC, Calabrese V, Elizan TS, Román GC, Sever JL (1988). Antibody to human and simian retrovirus, HTLV-1, HTLV-11, HIV, STLV-III, and SRV-I not increased in patients with multiple sclerosis. Ann Neurol [Suppl] 23:171–173
Mahnke C, Löchelt M, Bannert H, Flügel RM (1990) Specific enzymelinked immunosorbent assay for the detection of antibodies to the human spumavirus. J Virol Methods 29:13–22
Mahnke C, Kashaiya P, Rössler J, Bannert H, Levin A, Blattner WA, Dietrich M, Luande J, Löchelt M, Friedman-Klein E, Komaroff AL, Loh PC, Westarp M-E, Flügel RM (1992) Human spumavirus antibodies in sera from African patients. Arch Virol 123:243–253
Molin L, Ruhnek-Forsbeck M, Svennerholm B (1991) One year Acyclovir suppression of frequently recurring genital herpes: a study of efficacy, safety, virus sensitivity and antibody response. Scand J Infect 78:33–39
Ohta M, Ohta K, Mori F, Nishitani H, Saida T (1986) Sera from patients with multiple sclerosis react with human Tcell lymphotropic virus gag proteins but not env proteins — Western blotting analysis. J Immunol 137:3440–3443
Perron H, Geny C, Laurent A, Mouriquand C, Pellat J, Perret J, Seigneurin JM (1989) Leptomenigeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res Virol 140:551–561
Perron H, Lalande B, Gratacap B, Lauent A, Genoulaz O, Geny C, Mallaret M, Schuller E, Stoebner P, Seigneurin JM (1991) Isolation of retrovirus from patients with multiple sclerosis. Lancet 337:862–863
Pétursson G, Nathanson N, Georgsson G, Panitch H, Pálsson P (1976) Pathogenesis of visna. I. Sequential virologic, serologic and pathologic studies. Lab Invest 35:402–412
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
Pyykkö I, Vesanen M, Asikainen K, Koskiniemi M, Airaksinen L, Vaheri A (1993) Human spumaretrovirus in the etiology of sudden hearing loss. Acta Otolaryngol (Stockh) 113:109–112
Reddy EP, Sandberg-Wollheim M, Mettus RV, Ray PE, DeFreitas E, Koprowski H (1989) Amplification and molecular cloning of HTLV-I sequences from DNA of multiple sclerosis patients. Science 243:529–533
Resnick L, DiMarzo-Veronese F, Schüpbach J, Tourtellotte WW, Ho D, Müller F, Shapshak P, Vogt M, Groopman J, Markham P, Gallo R (1985) Intra-blood-brain-barrier synthesis of HTLV-III-specific IgG in patients with neurologic symptoms associated with AIDS or AIDS-related complex. J Engl J Med 313:1498–1510
Rogers NG, Basnight M, Gibbs CJ, Gajdusek DC (1967) Latent viruses in chimpanzees with experimental kuru. Nature 216:446–449
Román GC (1987) Retrovirus-associated myelopathies. Arch Neurol 44: 659–663
Rozenberg F, Lefebvre S, Lubetzki C, Lebon P, Lyon-Caen O, Brahic M, Bureau J-F (1991) Analysis of retroviral sequences in the spinal form of multiple sclerosis. Ann Neurol 29:333–336
Svenningsson A, Lycke J, Svennerholm B, Gronowitz S, Andersen O (1992) No evidence for spumavirus or oncovirus infection in relasping-remitting multiple sclerosis. Ann Neurol 32: 711–714
Tibbling G, Link H, Ohman S (1977) Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest 37:385–390
Wikkelsö C, Andersson M, Andersson R, Blomstrand C (1984) Isoelectric focusing followed by silver staining. Eur Neurol 23:306–312
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lycke, J., Svennerholm, B., Svenningsson, A. et al. Human spumaretrovirus antibody reactivity in multiple sclerosis. J Neurol 241, 204–209 (1994). https://doi.org/10.1007/BF00863769
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00863769