Summary
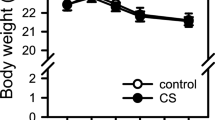
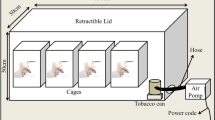
It is suggested that passive smoking or smoke-exposure increase the risk of coronary heart disease. The same mechanisms as active smoking might play a role. The aim of this study was to determine whether exposure to smoke aggravated ischaemia/reperfusion injury. As a parameter of cellular function and integrity mitochondrial oxidative function was measured. Low molecular weight iron (LMWI) and α-tocopherol levels were determined to assess the possibility of toxic hydroxyl radical involvement in myocardial ischaemia/reperfusion injury of smoke-exposed rats. Rats were exposed to a small concentration of cigarette smoke for 2 months (the carboxyhemoglobin concentration did not increase), whereafter hearts were isolated and subjected to ischaemia and ischaemia followed by reperfusion. Mitochondrial oxidative function, low molecular weight iron and α-tocopherol were determined. The impairment in mitochondrial oxidative function, LMWI content elevation and the decrease in α-tocopherol concentration during ischaemia/reperfusion were significantly more severe in hearts of smoke-exposed rats than non-smokers. These results suggest that exposure to smoke increased the sensitivity of hearts to ischaemia/reperfusion injury, and that a free radical mechanism might participate.
Similar content being viewed by others
References
Airriess GR, Changchit C, Chen LC, Chow CK (1988) Increased vitamin E levels in the lungs of guinea pigs exposed to mainstream or sidestream smoke. Nutrition Research 8:653–661
Dobson AJ, Alexander HM, Heller RF, Lloyd DM (1991) Passive smoking and the risk of heart attack or coronary death. Med J Aust 154:793–797
Elsayed NM, Mustafa MG, Mead JF (1990) Increased vitamin E content in the lung after ozone exposure: a possible mobilization in response to oxidative stress. Arch Biochem Biophys 282:263–269
Ferrari R, Ceconi C, Curello S, Cargnoni A, Pasini E, De Giuli F, Albertini A (1991) Role of oxygen free radicals in ischemic and reperfused myocardium. Am J Clin Nutr 53:2155–2225
Glantz SA, Parmley W (1991) Passive smoking and heart disease, epidemiology, physiology, and biochemistry. Circulation 83:1–12
Gvozdjàkovà A, Bada V, Sány L, Kucharská J, Kruty F, Bozek P, Trstansky L, Gvozdják J (1984) Smoke cardiomyopathy: disturbance of oxidative processed in myocardial mitochondria. Cardiovasc Res 18:229–232
Gvozdjàkovà A, Kucharská J, Sany L, Gvozdják J (1984) The effect of cigarette smoke on cytochrome-oxidase of the heart muscle. Cor Vasa 26:466–468
Halliwell B, Gutteridge JMC (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods in Enzymol 186:1–85
Humble C, Crofte J, Gerber A, Casper M, Hames CG, Tyroler HE (1990) Passive smoking and 20-year cardiovascular disease mortality among nonsmoking wives, Evans County, Georgia. Am J Public Health 80:599–601
Krause GS, Joyce KM, Nayini NR, Zonia CL, Garritano AM, Hoehner TJ (1983) Cardial arrest and resuscitation; brain iron delocalization during reperfusion. Ann Emerg Med 15:1037–1043
Krebs HA, Henseleit K (1932) Untersuchungen über die Harnstoff-Bildung im Tierkörper. Z Physiol Chem 210:33–66
Lochner A, van Niekerk I, Kotze JCN (1985) Normothermic ischemic cardiac arrest of the isolated perfused rat heart: effects of trifluoperazine and lysolecithin on mechanical and metabolic recovery. Basic Res Cardiol 780:363–376
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
McCusker K, Hoidal J (1990) Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamsters. Am Rev Respir Dis 141:678–682
Neely JR, Liebermeister H, Battersby EJ, Morgan HE (1974) Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212:804–814
Pryor WA, Church DF, Evans MD, Rice WY, Hayes JR (1990) A comparison of the free radical chemistry of tobacco-burning cigarettes and cigarettes that only heat tobacco. Free Rad Biol Med 8:275–279
Sordahl LA, Johnson C, Blailock ZR, Schwartz A (1971) The mitochondrion. Methods Pharmacol 1:247–286
Turnham DI, Smith E, Flora PG (1988) Concurrent liquid-chromatographic assay of retinol, α-tocopherol, β-carotene, α-carotene, lycopene and β-cryptoxanthin in plasma, with tocopherol acetate as internal standard. Clin Chem 34:377–381
Van Jaarsveld H, Lochner A (1982) Oxidative phosphorylation function of two mitochondrial preparations from heart: effects of ischaemia and cytochrome c. Basic Res Cardiol 77:388–403
Van Jaarsveld H, Potgieter GM, Kuyl JM, Barnard HC, Barnard SP (1990) The effect of desferal on rat heart mitochondrial function, iron content, and xanthine dehydrogenase/oxidase conversion during ischemia/reperfusion. Clin Biochem 23:509–513
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Jaarsveld, H., Kuyl, J.M. & Alberts, D.W. Exposure of rats to low concentration of cigarette smoke increases myocardial sensitivity to ischaemia/reperfusion. Basic Res Cardiol 87, 393–399 (1992). https://doi.org/10.1007/BF00796524
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00796524