Summary
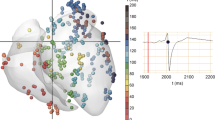
The goal of this study was to elucidate the causes why the proarrhythmic activity of sodium channel blocking drugs is enhanced during the post-infarction period. Therefore, we studied the effects of a reduction in sodium conductance on the action potential duration and its dispersion in a simulated array of 1600 ventricular myocytes. Cardiac tissue is known to possess anisotropic properties with regard to the intercellular electrical resistance (R). Infarction as well as aging causes deposition of collagen in the cardiac tissue, thereby inducing zones of high electrical resistance leading to a non-uniform anisotropy (Spach et al., Circ Res 62∶811, 1988). For our study an array of 40*40 ventricular myocytes was simulated using Beeler-Reuter-algorithms. Physical tissue properties were assumed to be either a) uniform anisotropic (i.e., all longitudinal R=5000 Ωcm, all transversal R=20000 Ωcm; UA) or b) non-uniform anisotropic (i.e., transversal R for the inner 10*10 cells was set to 1010 Ωcm; NUA). Mean action potential duration (APD) was increased under UA (287 ms, dispersion: 0,8 ms) when compared to NUA (285 ms, disp.: 3,2 ms). Assuming a 25% decrease in sodium conductance, we found the total activation time (TAT) to be increased (from 99 to 139 ms), indicating slowing of conduction, APD to be shortened (from 287 to 259 ms), and the APD-dispersion to be increased (from 0.8 to 29 ms) in UA. These changes were more pronounced in the case of NUA: increase in TAT from 103 to 150 ms, APD-shortening from 285 to 214 ms and a marked increase in APD-Dispersion from 3.2 to 53 ms). From these results it is concluded that a) the effects of a reduced sodium conductance are more pronounced in NUA tissue, and b) that the resulting increase in dispersion may provoke arrhythmia by local differences in APD.
This may be one of the mechanisms underlying the increased proarrhythmic risk of class I antiarrhythmic drugs in the postinfarction period.
Similar content being viewed by others
References
McAllister RE, Noble D, Tsien RW (1975) Reconstruction of the electrical activity of cardiac Purkinje fibers. J Physiol (London) 251:1–59
Barr RC, Plonsey, R (1984) Propagation of excitation in idealized anisotropic two-dimensional tissue. Biophys J 45:1191–1202
Beeler GW, Reuter H (1977) Reconstruction of the action potential of myocardial fibres. J Physiol (London) 268: 177–210
Cardiac arrhythmia suppression trial (CAST) investigators (1989) Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl J Med 321:393–398
Cole WC, Picone JB, Sperelakis N (1988) Gap junction uncoupling and discontinuous propagation in the heart: A comparison of experimental data with computer simulations. Biophys J 53:809–818
Crank J, Nicholson P (1947) A practical method for numerical evaluation of solutions of partial differential equations of the heat-conduction type. Proc Cambridge Phil Soc 43:50–67
DiFrancesco D, Noble D (1985) A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Trans R Soc Lond B 307:353–398
Dhein S, Müller A, Klaus W (1989) The proarrhythmic risk of flecainide, propafenone and lidocaine in isolated rabbit hearts. Med Sci Res 18:111–113
Dhein S, Müller A, Klaus W (1992) Comparative study on the proarrhythmic effects of some antiarrhythmic agents (submitted for publication)
Dhein S, Müller A, Klaus W (1990) Unterschiede in der arrhythmogenen Aktivität von Flecainid im Vergleich zu Lidocain: Neue Aspekte durch epikardiales Mapping. Z Kardiol 79, Suppl 1: 92
Echt DS et al (1991) Mortality and morbidity in patients receiving encainide, flecainide or placebo. The cardiac arrhythmia suppression trial. N Engl J Med 324:781–788
Eickhorn R, Weirich J, Hornung D, Antoni H (1990) Use dependence of sodium current inhibition by tetrodotoxin in rat cardiac muscle: influence of channel state. Pflüg Arch Eur J Physiol 416:398–405
Fenoglio JJ, Pham TD, Harken AH, Horowitz LN, Josephson ME, Wit AL (1983) Recurrent sustained ventricular tachycardia: Structure and ultrastructure of subendocardial region where tachycardia originates. Circulation68:518–533
Gardner PI, Ursell PC, Fenoglio JJ, Wit AL (1985) Electrophysiological and anatomic basis for fractionated electrograms from healed myocardial infarcts. Circulation 72:596–611
Han J, Moe GK (1964) Nonuniform recovery ofexcitability in ventricular muscle. Circ Res 16:46–60
Hondeghem LM, Katzung BG (1977) Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochimica et Biophysica Acta 472:373–398
Joyner RW, Westerfield M, Moore JW, Stockbridge N (1978) A numerical method to model excitable cells. Biophys J. 22:155–170
Karagueuzian HS, Singh BN, Mandel WJ (1987) Antiarrhythmic drugs: mode of action, pharmacokinetic properties and clinical applications. In: Mandel WJ (ed) Cardiac arrhythmias (2nd Edition). JB Lippincott Co, Philadelphia, pp 697–737
Kleber AG, Janse MJ, van Capelle FJL, Durrer D (1978) Mechanism and time course of ST and TQ segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings Circ Res 42:603–613
Kuo CS, Munakata K, Reddy CP, Surawitz B (1983) Characteristics and possible mechanisms of ventricular arrhythmias dependent on the dispersion of action potential duration. Circulation 67:1356–1367
Leon LJ, Roberg. 21. Leon LJ RobergerFA (1991) Directional characteristics of action potential propagation in cardiac muscle. A model study. Circ Res 69:378–395
Lesh MD, Pring M, Spear JF (1989) Cellular uncoupling can unmask dispersion of action potential duration in ventricular myocardium. A computer modeling study. Circ Res 65:1426–1440
Luo C, Rudy Y (1991) A model of the ventricular cardiac action potential. Depolarization, Repolarization, and their interaction. Circ Res 68:1501–1526
Merx W, Yoon MS, Han J (1977) The role of local disparity in conduction and recovery time on ventricular vulnerability to fibrillation. Am Heart J 94:603–610
Moore JW, Ramon F, Joyner RW (1975) Axon voltage-clamp simulations. I. Methods and tests. Biophys J 15:11–24
Press WH, Flannery BP, Teukolsky SA, Vetterling WT (1989) Numerical recipes in PASCAL. Cambridge University Press, Cambridge.
Roberge FA, Vinet A, Victorri B (1986) Reconstruction of propagated electrical activity with a two-dimensional model of anisotropic heart muscle. Circ Res 58:461–475
Rosen MR, Wit AL (1983) Electropharmacology of antiarrhythmic drugs. Am Heart J 106:829–839
Ross TF & Mandel WJ (1987) Invasive cardiac electrophysiologic testing. In: Mandel WJ (ed), Cardiac arrhythmias (2nd Edition). JB Lippincott Co, Philadelphia, pp 101–142
Rudy Y, Quan WL (1987) A model study of the effects of the discrete cellular structure on electrical propagation in cardiac tissue. Circ Res 61:815–823
Sharp GH, Joyner RW (1980) Simulated propagation of cardiac action potentials. Biophys J 31:403–424
Shrier A, Clay JR, Brochu RM (1990) Effects of tetrodotoxin on heart cell aggregates. Biophys J 58:623–629
Spach MS (1983) The discontinuous nature of electrical propagation in cardiac muscle. Annals of Biomedical Engineering 11:209–261
Spach MS, Kootsey JM (1983) The nature of electrical propagation in cardiac muscle. Am J Physiol 244:H3-H22
Spach MS, Miller WT, Miller-Jones E, Warren RB, Barr RC (1979) Extracellular potentials related to intracellular action potentials during impulse conduction in anisotropic canine cardiac muscle. Circ Res 45:188–204
Spach MS, Dolber PC (1986) Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Circ Res 58:356–371
Spach MS, Dolber PC, Anderson AW (1989) Multiple regional differences in cellular properties that regulate repolarization and contraction in the right atrium of adult and newborn dogs. Circ Res 65:1594–1611
Spach MS, Dolber PC, Heidlage JF (1989) Interaction of inhomogeneities of repolarization with anisotropic propagation in dog atria. A mechanism for both preventing and initiating reentry. Circ Res 65:1612–1631
Spear JF, Michelson EL, Moore EN (1983) Cellular electrophysiological characteristics of chronically infarcted myocardium in dogs susceptible to sustained ventricular tachyarrhythmias. J Am Coll Cardiol 1:1099–1110
Tan RC, Joyner RW (1990) Electrotonic influenceson action potentials from isolated ventricular cells. Circ Res 67:1071–1081
Tidball JG, Smith R, Shattock MJ, Bers DM (1988) Differences in action potential configuration in ventricular trabeculae correlate with differences in density of transverse tubule-sarcoplasmic reticulum couplings. J Mol Cell Cardiol 20:539–546
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Müller, A., Dhein, S. Sodium channel blockade enhances dispersion of the cardiac action potential duration. Basic Res Cardiol 88, 11–22 (1993). https://doi.org/10.1007/BF00788526
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00788526