Summary
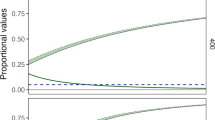
Green lichens have been shown to attain positive net photosynthesis in the presence of water vapour while blue-green lichens require liquid water (Lange et al. 1986). This behaviour is confirmed not only for species with differing photobionts in the genusPseudocyphellaria but for green and blue-green photobionts in a single joined thallus (photosymbiodeme), with a single mycobiont, and also when adjacent as co-primary photobionts. The different response is therefore a property of the photobiont. The results are consistent with published photosynthesis/water content response curves. The minimum thallus water content for positive net photosynthesis appears to be much lower in green lichens (15% to 30%, related to dry weight) compared to blue-greens (85% to 100%). Since both types of lichen rehydrate to about 50% water content by water vapour uptake only green lichens will show positive net photosynthesis. It is proposed that the presence of sugar alcohols in green algae allow them to retain a liquid pool (concentrated solution) in their chloroplasts at low water potentials and even to reform it by water vapour uptake after being dried. The previously shown difference in δ13C values between blue-green and green lichens is also retained in a photosymbiodeme and must be photobiont determined. The wide range of δ13C values in lichens can be explained by a C3 carboxylation system and the various effects of different limiting processes for photosynthetic CO2 fixation. If carboxylation is rate limiting, there will be a strong discrimination of13CO2, at high internal CO2 partial pressure. The resulting very low δ13C values (-31 to-35‰) have been found only in green lichens which are able to photosynthesize at low thallus water content by equilibraiton with water vapour. When the liquid phase diffusion of CO2 becomes more and more rate limiting and the internal CO2 pressure decreases, the13C content of the photosynthates increases and less negative δ13C values results, as are found for blue-green lichens.
Similar content being viewed by others
References
Ahmadjian V (1982) International Lichenological Newsletter 15:19
Brock TD (1975) Effect of water potential on aMicrocoleus (Cyanophyceae) from a desert crust. J Phycol 2:316–320
Brown DH, Rapsch S, Beckett A, Ascaso C (1987) The effect of desiccation on cell shape in the lichenParmelia sulcata Taylor. New Phytol 195:295–300
Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z (1982) Effect of salinity and humidity on δ13C value of halophytes-evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia (Berlin) 52:121–124
Farrar JF, Smith DC (1976) Ecological physiology of the lichenHypogymnia physodes. III. The importance of the rewetting phase. New Phytol 77:115–125
Green TGA, Snelgar WP (1981) Carbon dioxide exchange in lichens: partition of total CO2 resistances at different thallus water contents into transport and carboxylation components. Physiol Plant 52:411–416
Green TGA, Snelgar WP, Wilkins AL (1985) Photosynthesis, water relations and thallus structure of Stictaceae lichens. In: Brown DH (ed) Lichen physiology and cell biology. Plenum Press, New York, pp 57–76
Green TGA, Henskens F, Wilkins AL (1987) Evidence for green and cyanobacterial co-primary photobionts in the lichen genusPseudocyphellaria. XIV International Botnaical Congress, Abstracts, 6-29-5:378
Hawksworth DL, Hill DJ (1984) The lichen-forming fungi. Blackie, Glasgow London
Hellebust JA (1976) Osmoregulation. Annu Rev Plant Physiol 27:485–506
James PW, Henssen A (1976) The morphological and taxonomic significance of cephalodia. In: Brown DH, Hawksworth DL, Bailey EH (eds) Lichenology: Progress and problems. Academic Press, London New York, pp 27–79
Kershaw KA (1977) Physiological-environmental interactions in lichens. II. The pattern of net photosynthetic acclimation inPeltigera canina (L.). Willd. var.praetextata (Floerke in Somm.) Hue, andPeltigera polydactyla (Neck.) Hoffm. New Phytol 79:377–390
Kershaw KA, MacFarlane JD (1982) Physiological-environmental interactions in lichens. XIII. Seasonal constancy of nitrogenase activity, net photosynthesis and respiration inCollema furfuraceum (Arn.) DR. New Phytol 90:723–734
Lange OL, Kilian E (1985) Reaktivierung der Photosynthese trokkener Flechten durch Wasserdampfaufnahme aus dem Luftraum: Artspezifisch unterschiedliches Verhalten. Flora (Jena) 176:7–23
Lange OL, Tenhunen JD (1981) Moisture content and CO2 exchange of lichens. II. Depression of net photosynthesis inRamalina maciformis at high water content is caused by increased thallus carbon dioxide diffusion resistance. Oecologia (Berlin) 51:426–429
Lange OL, Ziegler H (1986) Different limiting processes of photosynthesis in lichens. In: Marcelle R, Cljisters H, Van Poucke M (eds) Biological control of photosynthesis. Nijhoff, Dordrecht, pp 147–161
Lange OL, Kilian E, Ziegler H (1986) Water vapor uptake and photosynthesis of lichens: performance differences in species with green and blue-green algae as phycobionts. Oecologia (Berlin) 71:104–110
Larson DW (1979) Lichen water relations under drying conditions. New Phytol 82:713–731
Larson DW, Kershaw KA (1976) Studies on lichen dominated ecosystems. XVIII. Morphological control of evaporation in lichens. Can J Bot 54:2061–2073
MacFarlane JD, Kershaw KA (1977) Physiological-environmental interactions in lichens. IV. Seasonal changes in the nitrogenase activity ofPeltigera canina (L.) Willd. var.praetextata (Floerke in Somm.) Hue andPeltigera canina (L.) Willd. var.rufescens (Weiss) Mudd. New Phytol 79:403–408
Mazur P (1968) Survival of fungi after freezing and desiccation. In: Ainsworth GC, Sussman AL (eds) The fungi, vol 3. Academic Press, London, pp 325–394
Nobel PS (1983) Introduction to biophysical plant physiology. W.A. Freeman, San Francisco
Osmond CB, Ziegler H, Stichler W, Trimborn P (1975) Carbon isotope discrimination in alpine succulent plants supposed to be cabable of Crassulacean acid metabolism (CAM). Oecologia (Berlin) 18:209–217
Parker J (1968) Drought resistance mechanisms. In: Kozlowski TT (ed) Water defficits and plant growth, vol I. Academic Press, London, pp 195–234
Renner B, Galloway DJ (1982) Phycosymbiodemes inPseudocyphellaria in New Zealand. Mycotaxon 16:197–231
Smith DC, Galloway DJ (1982) Phycosymbiodemes inPseudocyphellaria in New Zealand. Mycotaxon 16:197–231
Smith DC, Molesworth S (1973) Lichen physiology. XIII. Effects of rewetting dry lichens. New Phytol 72:525–533
Snelgar WP, Green TGA (1981) Ecologically linked variation in morphology, acetylene reduction and water relations inPseudocyphellaria dissimilis. New Phytol 87:403–411
Snelgar WP, Brown DH, Green TGA (1980) A provisional survey of the interaction between net photosynthetic rate, respiratory rate and thallus water content in some New Zealand cryptogams. NZ J Bot 18:247–256
Snelgar WP, Green TGA, Wilkins AL (1981) Carbon dioxide exchange in lichens: resistances to CO2 uptake at different thallus water contents. New Phytol 88:353–361
Tonberg T, Holtan-Hartwig J (1983) Phycotype pairs inNephroma, Peltigera andLobaria in Norway. Nord J Bot 3:681–688
Vogel JC (1980) Fractionation of carbon isotopes during photosynthesis. Sitzungsber Heidelberger Akad Wiss, Mathem.-naturw. Klasse. Jg. 1980. Springer, Berlin Heidelberg New York
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Webb SJ (1965) Bound water in biological integrity. CC Thomas, Springfield Illinois
Wirth V (1983) Phytosoziologie, Ökologie und Systematik bei Flechten. Ber Dtsch Bot Ges 96:103–115
Zukal H (1895) Morphologische und biologische Untersuchungen über die Flecten. II. Sitzungsber k Akad Wiss Wien, Math. nat.cl. CIV pp 1303–1395
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lange, O.L., Green, T.G.A. & Ziegler, H. Water status related photosynthesis and carbon isotope discrimination in species of the lichen genusPseudocyphellaria with green or blue-green photobionts and in photosymbiodemes. Oecologia 75, 494–501 (1988). https://doi.org/10.1007/BF00776410
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00776410