Abstract
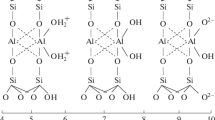
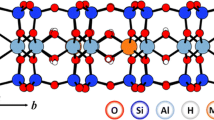
Controlled calcination of ion-exchanged Texas montmorillonite leads to layer charge reduction. Detailed chemical analyses of both exchangeable and unexchangeable metal species lead to the conclusion that, in complete contrast to the situation with Wyoming bentonite, Ni2+, Co2+ and Zn2+ are all capable of migrating from the interlamellar space into the octahedral region of the sheets where they are (i) isomorphously exchanged for Mg2+, and to a proportionately smaller extent, for Fe2+, (ii) trapped in the octahedral vacancies. In addition, they are converted to unexchangeable species on the interlamellar sheet surfaces by high temperature hydrolysis leading to hydroxide or oxide formation. It is suggested that protons within the octahedral region are bound as -OH+ 2rather than simply physically trapped.
Similar content being viewed by others
References
R.L. Cook, J. Am. Soc. Agron. 27 (1935) 297.
R. Greeve-Kelly, Clay Min. Bull. 2 (1953) 52.
R. Chaminade, Acad. Sci. Paris 254 (1962) 902.
J. Williams, J.H. Purnell and J.A. Ballantine, Catal. Lett. 9 (1991) 115.
J.H. Purnell, J. Williams and Lu Yun, Catal. Lett. 10 (1991) 63.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Purnell, J.H., Yun, L. Ionic migration and charge reduction in Ni2+-, Co2+- and Zn2+-exchanged Texas montmorillonite. Catal Lett 18, 235–241 (1993). https://doi.org/10.1007/BF00769442
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00769442